Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide
- PMID: 20185596
- PMCID: PMC2863977
- DOI: 10.2215/CJN.05380709
Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide
Abstract
Background and objectives: No medical treatment is available for polycystic liver disease, a frequent manifestation of autosomal-dominant polycystic kidney disease (ADPKD). In a prospective, randomized, double-blind, crossover study, 6 months of octreotide (40 mg every 28 days) therapy limited kidney volume growth more effectively than placebo in 12 patients with ADPKD.
Design, setting, participants, & measurements: In this secondary, post hoc analysis of the above study, octreotide-induced changes in liver volumes compared with placebo and the relationship between concomitant changes in liver and kidney volumes were evaluated. Those analyzing liver and kidney volumes were blinded to treatment.
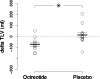
Results: Liver volumes significantly decreased from 1595 +/- 478 ml to 1524 +/- 453 ml with octreotide whereas they did not appreciably change with placebo. Changes in liver volumes were significantly different between the two treatment periods (-71 +/- 57 ml versus +14 +/- 85 ml). Octreotide-induced liver volume reduction was fully explained by a reduction in parenchyma volume from 1506 +/- 431 ml to 1432 +/- 403 ml. Changes in liver volumes were significantly correlated with concomitant changes in kidney volumes (r = 0.67) during octreotide but not during placebo treatment. Liver and kidney volume changes significantly differed with both treatments (octreotide: -71 +/- 57 ml versus +71 +/- 107; placebo: +14 +/- 85 ml versus +162 +/- 114), but net reductions in liver (-85 +/- 103 ml) and kidney (-91 +/- 125 ml) volume growth on octreotide versus placebo were similar.
Conclusions: Octreotide therapy reduces liver volumes in patients with ADPKD and is safe.
Figures


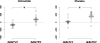
References
-
- Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287– 1301, 2007 - PubMed
-
- Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF, Jr, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiological Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1: 64– 69, 2006 - PubMed
-
- Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G: cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528– 537, 2004 - PubMed
-
- Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF: Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology 132: 1104– 1116, 2007 - PubMed
-
- Tack J, Carethers JM: This month in gastroenterology. Gastroenterology 132: 831– 834, 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

