A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis
- PMID: 20185723
- PMCID: PMC4824053
- DOI: 10.1126/science.1184096
A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis
Abstract
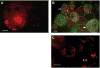
The nitrogen-fixing symbiosis between Sinorhizobium meliloti and its leguminous host plant Medicago truncatula occurs in a specialized root organ called the nodule. Bacteria that are released into plant cells are surrounded by a unique plant membrane compartment termed a symbiosome. We found that in the symbiosis-defective dnf1 mutant of M. truncatula, bacteroid and symbiosome development are blocked. We identified the DNF1 gene as encoding a subunit of a signal peptidase complex that is highly expressed in nodules. By analyzing data from whole-genome expression analysis, we propose that correct symbiosome development in M. truncatula requires the orderly secretion of protein constituents through coordinated up-regulation of a nodule-specific pathway exemplified by DNF1.
Figures



References
-
- Thompson AR, Vierstra RD. Curr Opin Plant Biol. 2005;8:165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

