Latent herpesvirus infection augments experimental pulmonary fibrosis
- PMID: 20185751
- PMCID: PMC2830399
- DOI: 10.1164/rccm.200905-0798OC
Latent herpesvirus infection augments experimental pulmonary fibrosis
Abstract
Rationale: No effective treatment exists for idiopathic pulmonary fibrosis, and its pathogenesis remains unclear. Accumulating evidence implicates herpesviruses as cofactors (either initiating or exacerbating agents) of fibrotic lung disease, but a role for latent herpesvirus infection has not been studied.
Objectives: To develop a murine model to determine whether latent herpesvirus infection can augment fibrotic responses and to gain insight into potential mechanisms of enhanced fibrogenesis.
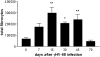
Methods: Mice were infected with murine gammaherpesvirus 14 to 70 days before a fibrotic challenge with fluorescein isothiocyanate or bleomycin so that the virus was latent at the time of fibrotic challenge. Measurements were made after viral infection alone or after the establishment of fibrosis.
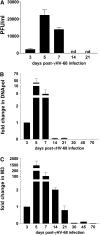
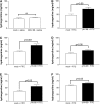
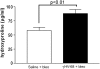
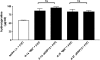
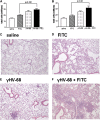
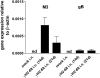
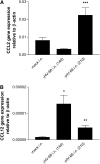
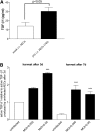
Measurements and main results: gammaHerpesvirus is latent by 14 days post infection, and infection 14 to 70 days before fibrotic challenge augmented fibrosis. Fibrotic augmentation was not dependent on reactivation of the latent virus to a lytic state. Total cell numbers and fibrocyte numbers were increased in the lungs of latently infected mice administered fibrotic challenge compared with mock-infected mice that received fibrotic challenge. Latent infection up-regulates expression of proinflammatory chemokines, transforming growth factor-beta1, and cysteinyl leukotrienes in alveolar epithelial cells.
Conclusions: Latent gammaherpesvirus infection augments subsequent fibrotic responses in mice. Enhanced fibrosis is associated with the induction of profibrotic factors and the recruitment of fibrocytes. Our data complement existing human and animal data supporting the hypothesis that gammaherpesviruses can serve as initiating cofactors in the pathogenesis of pulmonary fibrosis.
Figures
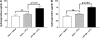
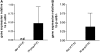




References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. - PubMed
-
- Lok SS, Stewart JP, Kelly BG, Hasleton PS, Egan JJ. Epstein-Barr virus and wild p53 in idiopathic pulmonary fibrosis. Respir Med 2001;95:787–791. - PubMed
-
- Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:510–513. - PubMed
-
- Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

