Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis
- PMID: 20185764
- PMCID: PMC2850237
- DOI: 10.1210/en.2009-1180
Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis
Abstract
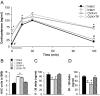
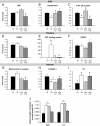
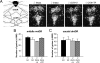
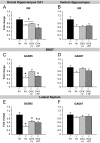
Appropriate interactions between serotonin (5-HT) and stress pathways are critical for maintaining homeostasis. Dysregulation of hypothalamic-pituitary-adrenal (HPA) stress axis is a common feature in affective disorders in which an involvement of 5-HT neurocircuitry has been implicated in disease vulnerability and treatment responsiveness. Because there is a greater prevalence of affective disorders in women, sex differences in the 5-HTergic influence on stress pathways may contribute to disease disparity. Therefore, our studies compared stress or citalopram-induced corticosterone levels in male and female mice. To determine whether sex-dependent HPA axis responsiveness was mediated by the difference in testosterone levels, testosterone-treated females were also examined. Gene expression patterns in 5-HTergic and stress neurocircuitry were analyzed to determine sites of potential sex differences and mechanisms of testosterone action. As expected, restraint stress corticosterone levels were higher in intact females and were masculinized by testosterone. Interestingly, citalopram administration independent of stress resulted in a greater corticosterone response in females, which was also masculinized by testosterone. Analyses along the 5-HT-HPA axis revealed sex differences including greater pituitary 5-HT receptors and adrenal weights in females. Moreover, in stress-regulatory regions, we found sex differences in glucocorticoid receptor and glutamic acid decarboxylase expression supportive of greater inhibitory modulation and feedback potential in males. Taken together, these data suggest that multiple sites related to 5-HTergic stimulation, corticosterone production, and negative feedback of HPA neurocircuitry combine to produce higher female stress responsiveness. These studies support a potential for sex-specific involvement of 5-HT and stress pathways in the etiology of affective disorders.
Figures

Similar articles
-
Androgenic influence on serotonergic activation of the HPA stress axis.Endocrinology. 2011 May;152(5):2001-10. doi: 10.1210/en.2010-0964. Epub 2011 Mar 8. Endocrinology. 2011. PMID: 21385938 Free PMC article.
-
Treatment with an SSRI antidepressant restores hippocampo-hypothalamic corticosteroid feedback and reverses insulin resistance in low-birth-weight rats.Am J Physiol Endocrinol Metab. 2010 May;298(5):E920-9. doi: 10.1152/ajpendo.00606.2009. Epub 2010 Jan 26. Am J Physiol Endocrinol Metab. 2010. PMID: 20103738 Free PMC article.
-
Sex differences in serotonin (5-HT) 1A receptor regulation of HPA axis and dorsal raphe responses to acute restraint.Psychoneuroendocrinology. 2014 Feb;40:232-41. doi: 10.1016/j.psyneuen.2013.11.020. Epub 2013 Dec 1. Psychoneuroendocrinology. 2014. PMID: 24485495
-
Dissection of glucocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis by gene targeting in mice.Front Neuroendocrinol. 2015 Jan;36:150-64. doi: 10.1016/j.yfrne.2014.09.002. Epub 2014 Sep 27. Front Neuroendocrinol. 2015. PMID: 25256348 Free PMC article. Review.
-
Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity.Stress. 2017 Sep;20(5):476-494. doi: 10.1080/10253890.2017.1369523. Epub 2017 Aug 31. Stress. 2017. PMID: 28859530 Free PMC article. Review.
Cited by
-
Serotonergic effects on feeding, but not hypothalamus-pituitary-adrenal secretion, are altered in ovine pregnancy.Am J Physiol Endocrinol Metab. 2012 May 1;302(10):E1231-8. doi: 10.1152/ajpendo.00582.2011. Epub 2012 Feb 28. Am J Physiol Endocrinol Metab. 2012. PMID: 22374755 Free PMC article.
-
Sex Differences in Physiological Acclimatization after Transfer in Wistar Rats.Animals (Basel). 2014 Oct 30;4(4):693-711. doi: 10.3390/ani4040693. Animals (Basel). 2014. PMID: 26479007 Free PMC article.
-
Drosophila melanogaster as a neurobehavioral model for sex differences in stress response.Front Behav Neurosci. 2025 Jul 11;19:1581763. doi: 10.3389/fnbeh.2025.1581763. eCollection 2025. Front Behav Neurosci. 2025. PMID: 40718064 Free PMC article. Review.
-
Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice.Behav Brain Res. 2011 Jan 1;216(1):349-57. doi: 10.1016/j.bbr.2010.08.019. Epub 2010 Aug 21. Behav Brain Res. 2011. PMID: 20732356 Free PMC article.
-
Rapid hypothalamic-pituitary recovery after chronic glucocorticoid therapy enables strategies that prevent adrenal suppression.bioRxiv [Preprint]. 2025 May 4:2025.04.30.651350. doi: 10.1101/2025.04.30.651350. bioRxiv. 2025. PMID: 40655007 Free PMC article. Preprint.
References
-
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB 1999 The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12 - PubMed
-
- Kendler KS, Karkowski LM, Prescott CA 1999 Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–841 - PubMed
-
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS 1994 Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19 - PubMed
-
- Cowen PJ, Parry-Billings M, Newsholme EA 1989 Decreased plasma tryptophan levels in major depression. J Affect Disord 16:27–31 - PubMed
-
- Owens MJ, Nemeroff CB 1994 Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 40:288–295 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

