A novel mitochondrial K(ATP) channel assay
- PMID: 20185796
- PMCID: PMC2857559
- DOI: 10.1161/CIRCRESAHA.109.215400
A novel mitochondrial K(ATP) channel assay
Abstract
Rationale: The mitochondrial ATP sensitive potassium channel (mK(ATP)) is implicated in cardioprotection by ischemic preconditioning (IPC), but the molecular identity of the channel remains controversial. The validity of current methods to assay mK(ATP) activity is disputed.
Objective: We sought to develop novel methods to assay mK(ATP) activity and its regulation.
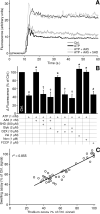
Methods and results: Using a thallium (Tl(+))-sensitive fluorophore, we developed a novel Tl(+) flux based assay for mK(ATP) activity, and used this assay probe several aspects of mK(ATP) function. The following key observations were made. (1) Time-dependent run down of mK(ATP) activity was reversed by phosphatidylinositol-4,5-bisphosphate (PIP(2)). (2) Dose responses of mK(ATP) to nucleotides revealed a UDP EC(50) of approximately 20 micromol/L and an ATP IC(50) of approximately 5 micromol/L. (3) The antidepressant fluoxetine (Prozac) inhibited mK(ATP) (IC(50)=2.4 micromol/L). Fluoxetine also blocked cardioprotection triggered by IPC, but did not block protection triggered by a mK(ATP)-independent stimulus. The related antidepressant zimelidine was without effect on either mK(ATP) or IPC.
Conclusions: The Tl(+) flux mK(ATP) assay was validated by correlation with a classical mK(ATP) channel osmotic swelling assay (R(2)=0.855). The pharmacological profile of mK(ATP) (response to ATP, UDP, PIP(2), and fluoxetine) is consistent with that of an inward rectifying K(+) channel (K(IR)) and is somewhat closer to that of the K(IR)6.2 than the K(IR)6.1 isoform. The effect of fluoxetine on mK(ATP)-dependent cardioprotection has implications for the growing use of antidepressants in patients who may benefit from preconditioning.
Figures


References
-
- Auchampach JA, Grover GJ, Gross GJ. Blockade of ischaemic preconditioning in dogs by the novel ATP dependent potassium channel antagonist sodium 5-hydroxydecanoate. Cardiovasc Res. 1992;26:1054–62. - PubMed
-
- Schultz JE, Qian YZ, Gross GJ, Kukreja RC. The ischemia-selective KATP channel antagonist, 5-hydroxydecanoate, blocks ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 1997;29:1055–60. - PubMed
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

