Variants in ASK1 are associated with skeletal muscle ASK1 expression, in vivo insulin resistance, and type 2 diabetes in Pima Indians
- PMID: 20185809
- PMCID: PMC2857909
- DOI: 10.2337/db09-1700
Variants in ASK1 are associated with skeletal muscle ASK1 expression, in vivo insulin resistance, and type 2 diabetes in Pima Indians
Abstract
Objective: Prior genome-wide association and exon array expression studies both provided suggestive evidence that apoptosis signal regulating kinase 1 (ASK1) may influence in vivo insulin action in Pima Indians. Genetic variants in or near ASK1 were analyzed to assess the role of this gene in insulin action and type 2 diabetes.
Research design and methods: Genotypic data from 31 variants were used to determine the linkage disequilibrium pattern across ASK1 in Pima Indians. Eight tag SNPs were initially genotyped in 3,501 full-heritage Pima Indians. Replication for association with diabetes was assessed in a second population-based sample of 3,723 Native Americans and the published DIAGRAM study. Quantitative traits were analyzed in 536 nondiabetic Native Americans, and ASK1 expression was examined in skeletal muscle of 153 nondiabetic Native Americans.
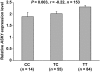
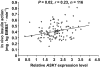
Results: Three tag SNPs were associated with type 2 diabetes (rs35898099, P = 0.003, odds ratio [95% CI] 1.27 [1.08-1.47]; rs1570056, P = 0.007, 1.19 [1.05-1.36]; rs7775356, P = 0.04, 1.14 [1.01-1.28]) in the full-heritage Pima Indians. The association with rs35898099 was replicated in a second sample of Native Americans (P = 0.04, 1.22 [1.01-1.47]), while that for rs1570056 was replicated in the DIAGRAM study of Caucasians (Z statistic based P = 0.026; fixed-effect model, 1.06 [1.00-1.12]). The diabetes risk allele for rs1570056 was associated with reduced insulin action as assessed by either HOMA-IR in 2,549 nondiabetic full-heritage Pima Indians (P = 0.027) or a hyperinsulinemic-euglycemic clamp among 536 nondiabetic Native Americans (P = 0.02). Real-time PCR identified a positive correlation between ASK1 expression in skeletal muscle biopsies and in vivo insulin action (P = 0.02, r = 0.23), and the risk allele for rs1570056 was associated with lower ASK1 expression (P = 0.003, r = -0.22).
Conclusions: ASK1 variants may increase susceptibility to type 2 diabetes by decreasing insulin sensitivity via reduced ASK1 expression.
Figures

References
-
- Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol 1978;108:497–505 - PubMed
-
- Bogardus C. Metabolic abnormalities in the development of non-insulin dependent mellitus. In Diabetes Mellitus: A Fundamental and Clinical Text. LeRoith D, Taylor SI, Olefsky JM. Eds. Philadelphia, Lippincott Williams & Wilkins, 1996, p. 459–467
-
- Ravussin E, Bogardus C. Energy expenditure in the obese: is there a thrifty gene? Infusionstherapie 1990;17:108–112 - PubMed
-
- World Health Organization. Report of a WHO Study Group: Diabetes Mellitus Geneva, World Health Org., 1985. (Tech. Rep. Ser., no. 727)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

