Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo
- PMID: 20185816
- PMCID: PMC2874690
- DOI: 10.2337/db09-1625
Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo
Abstract
Objective: Insulin-mediated suppression of hepatic glucose production (HGP) is associated with sensitive intracellular signaling and molecular inhibition of gluconeogenic (GNG) enzyme mRNA expression. We determined, for the first time, the time course and relevance (to metabolic flux) of these molecular events during physiological hyperinsulinemia in vivo in a large animal model.
Research design and methods: 24 h fasted dogs were infused with somatostatin, while insulin (basal or 8 x basal) and glucagon (basal) were replaced intraportally. Euglycemia was maintained and glucose metabolism was assessed using tracer, (2)H(2)O, and arterio-venous difference techniques. Studies were terminated at different time points to evaluate insulin signaling and enzyme regulation in the liver.
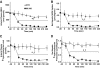
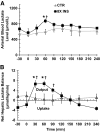
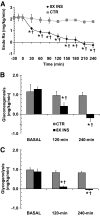
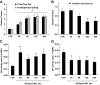
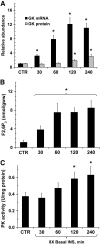
Results: Hyperinsulinemia reduced HGP due to a rapid transition from net glycogen breakdown to synthesis, which was associated with an increase in glycogen synthase and a decrease in glycogen phosphorylase activity. Thirty minutes of hyperinsulinemia resulted in an increase in phospho-FOXO1, a decrease in GNG enzyme mRNA expression, an increase in F2,6P(2), a decrease in fat oxidation, and a transient decrease in net GNG flux. Net GNG flux was restored to basal by 4 h, despite a substantial reduction in PEPCK protein, as gluconeogenically-derived carbon was redirected from lactate efflux to glycogen deposition.
Conclusions: In response to acute physiologic hyperinsulinemia, 1) HGP is suppressed primarily through modulation of glycogen metabolism; 2) a transient reduction in net GNG flux occurs and is explained by increased glycolysis resulting from increased F2,6P(2) and decreased fat oxidation; and 3) net GNG flux is not ultimately inhibited by the rise in insulin, despite eventual reduction in PEPCK protein, supporting the concept that PEPCK has poor control strength over the gluconeogenic pathway in vivo.
Figures


References
-
- Adkins A, Basu R, Persson M, Dicke B, Shah P, Vella A, Schwenk WF, Rizza R: Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in nondiabetic humans. Diabetes 2003; 52: 2213–2220 - PubMed
-
- Boden G, Cheung P, Homko C: Effects of acute insulin excess and deficiency on gluconeogenesis and glycogenolysis in type 1 diabetes. Diabetes 2003; 52: 133–137 - PubMed
-
- Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M: FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab 2002; 283: E12–E19 - PubMed
-
- Cherrington AD: Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999; 48: 1198–1214 - PubMed
-
- Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD: Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 2001; 50: 1872–1882 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

