A role for huntington disease protein in dendritic RNA granules
- PMID: 20185826
- PMCID: PMC2857123
- DOI: 10.1074/jbc.M110.114561
A role for huntington disease protein in dendritic RNA granules
Abstract
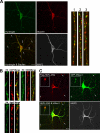
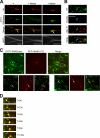
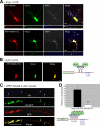
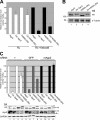
Regulated transport and local translation of mRNA in neurons are critical for modulating synaptic strength, maintaining proper neural circuitry, and establishing long term memory. Neuronal RNA granules are ribonucleoprotein particles that serve to transport mRNA along microtubules and control local protein synthesis in response to synaptic activity. Studies suggest that neuronal RNA granules share similar structures and functions with somatic P-bodies. We recently reported that the Huntington disease protein huntingtin (Htt) associates with Argonaute (Ago) and localizes to cytoplasmic P-bodies, which serve as sites of mRNA storage, degradation, and small RNA-mediated gene silencing. Here we report that wild-type Htt associates with Ago2 and components of neuronal granules and co-traffics with mRNA in dendrites. Htt was found to co-localize with RNA containing the 3'-untranslated region sequence of known dendritically targeted mRNAs. Knockdown of Htt in neurons caused altered localization of mRNA. When tethered to a reporter construct, Htt down-regulated reporter gene expression in a manner dependent on Ago2, suggesting that Htt may function to repress translation of mRNAs during transport in neuronal granules.
Figures

Similar articles
-
Huntingtin mediates dendritic transport of β-actin mRNA in rat neurons.Sci Rep. 2011;1:140. doi: 10.1038/srep00140. Epub 2011 Nov 3. Sci Rep. 2011. PMID: 22355657 Free PMC article.
-
Cataloguing and Selection of mRNAs Localized to Dendrites in Neurons and Regulated by RNA-Binding Proteins in RNA Granules.Biomolecules. 2020 Jan 22;10(2):167. doi: 10.3390/biom10020167. Biomolecules. 2020. PMID: 31978946 Free PMC article. Review.
-
Huntington's disease protein contributes to RNA-mediated gene silencing through association with Argonaute and P bodies.Proc Natl Acad Sci U S A. 2008 Aug 5;105(31):10820-5. doi: 10.1073/pnas.0800658105. Epub 2008 Jul 31. Proc Natl Acad Sci U S A. 2008. PMID: 18669659 Free PMC article.
-
An RNA-interacting protein, SYNCRIP (heterogeneous nuclear ribonuclear protein Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites.J Biol Chem. 2004 Dec 17;279(51):53427-34. doi: 10.1074/jbc.M409732200. Epub 2004 Oct 8. J Biol Chem. 2004. PMID: 15475564
-
mRNPs, polysomes or granules: FMRP in neuronal protein synthesis.Curr Opin Neurobiol. 2006 Jun;16(3):265-9. doi: 10.1016/j.conb.2006.05.010. Epub 2006 May 16. Curr Opin Neurobiol. 2006. PMID: 16707258 Review.
Cited by
-
Intracellular and intercellular transport of RNA organelles in CXG repeat disorders: The strength of weak ties.Front Mol Biosci. 2022 Dec 16;9:1000932. doi: 10.3389/fmolb.2022.1000932. eCollection 2022. Front Mol Biosci. 2022. PMID: 36589236 Free PMC article. Review.
-
Protein Kinase CK2 and Its Potential Role as a Therapeutic Target in Huntington's Disease.Biomedicines. 2022 Aug 15;10(8):1979. doi: 10.3390/biomedicines10081979. Biomedicines. 2022. PMID: 36009526 Free PMC article. Review.
-
MicroRNAs located in the Hox gene clusters are implicated in huntington's disease pathogenesis.PLoS Genet. 2014 Feb 27;10(2):e1004188. doi: 10.1371/journal.pgen.1004188. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586208 Free PMC article.
-
Lost in Translation: Evidence for Protein Synthesis Deficits in ALS/FTD and Related Neurodegenerative Diseases.Adv Neurobiol. 2018;20:283-301. doi: 10.1007/978-3-319-89689-2_11. Adv Neurobiol. 2018. PMID: 29916024 Free PMC article. Review.
-
Quantitative Proteomic Analysis Reveals Similarities between Huntington's Disease (HD) and Huntington's Disease-Like 2 (HDL2) Human Brains.J Proteome Res. 2016 Sep 2;15(9):3266-83. doi: 10.1021/acs.jproteome.6b00448. Epub 2016 Aug 3. J Proteome Res. 2016. PMID: 27486686 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

