Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients
- PMID: 20186122
- PMCID: PMC2857464
- DOI: 10.1038/emboj.2010.21
Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients
Abstract
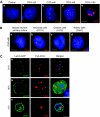
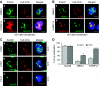
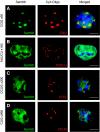
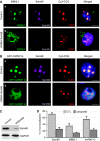
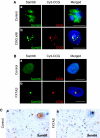
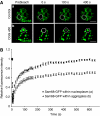
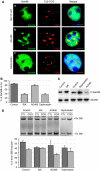
Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) is a neurodegenerative disorder caused by expansion of 55-200 CGG repeats in the 5'-UTR of the FMR1 gene. FXTAS is characterized by action tremor, gait ataxia and impaired executive cognitive functioning. It has been proposed that FXTAS is caused by titration of RNA-binding proteins by the expanded CGG repeats. Sam68 is an RNA-binding protein involved in alternative splicing regulation and its ablation in mouse leads to motor coordination defects. Here, we report that mRNAs containing expanded CGG repeats form large and dynamic intranuclear RNA aggregates that recruit several RNA-binding proteins sequentially, first Sam68, then hnRNP-G and MBNL1. Importantly, Sam68 is sequestered by expanded CGG repeats and thereby loses its splicing-regulatory function. Consequently, Sam68-responsive splicing is altered in FXTAS patients. Finally, we found that regulation of Sam68 tyrosine phosphorylation modulates its localization within CGG aggregates and that tautomycin prevents both Sam68 and CGG RNA aggregate formation. Overall, these data support an RNA gain-of-function mechanism for FXTAS neuropathology, and suggest possible target routes for treatment options.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
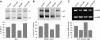

References
-
- Argentini M, Strub JM, Carapito C, Sanglier S, Van-Dorsselaer A (2008) An optimized MALDI mass spectrometry method for improved detection of lysine/arginine/histidine free peptides. J Proteome Res 7: 5062–5069 - PubMed
-
- Arocena DG, Iwahashi CK, Won N, Beilina A, Ludwig AL, Tassone F, Schwartz PH, Hagerman PJ (2005) Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG repeat RNA in human cultured neural cells. Human Mol Genet 14: 3661–3671 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

