Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli
- PMID: 20186264
- PMCID: PMC2826370
- DOI: 10.1371/journal.pbio.1000317
Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli
Abstract
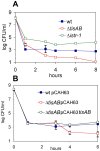
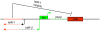
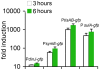
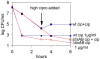
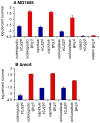
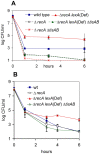
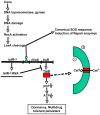
Bacteria induce stress responses that protect the cell from lethal factors such as DNA-damaging agents. Bacterial populations also form persisters, dormant cells that are highly tolerant to antibiotics and play an important role in recalcitrance of biofilm infections. Stress response and dormancy appear to represent alternative strategies of cell survival. The mechanism of persister formation is unknown, but isolated persisters show increased levels of toxin/antitoxin (TA) transcripts. We have found previously that one or more components of the SOS response induce persister formation after exposure to a DNA-damaging antibiotic. The SOS response induces several TA genes in Escherichia coli. Here, we show that a knockout of a particular SOS-TA locus, tisAB/istR, had a sharply decreased level of persisters tolerant to ciprofloxacin, an antibiotic that causes DNA damage. Step-wise administration of ciprofloxacin induced persister formation in a tisAB-dependent manner, and cells producing TisB toxin were tolerant to multiple antibiotics. TisB is a membrane peptide that was shown to decrease proton motive force and ATP levels, consistent with its role in forming dormant cells. These results suggest that a DNA damage-induced toxin controls production of multidrug tolerant cells and thus provide a model of persister formation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Del Pozo J, Patel R. The challenge of treating biofilm-associated bacterial infections. Clinical Pharmacol Ther. 2007;82:204–209. - PubMed
-
- Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. - PubMed
-
- Bigger J. W. Treatment of staphylococcal infections with penicillin. Lancet. 1944;2:497–500.
-
- Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett. 2004;230:13–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

