Effects of intermittent IL-2 alone or with peri-cycle antiretroviral therapy in early HIV infection: the STALWART study
- PMID: 20186278
- PMCID: PMC2826395
- DOI: 10.1371/journal.pone.0009334
Effects of intermittent IL-2 alone or with peri-cycle antiretroviral therapy in early HIV infection: the STALWART study
Abstract
Background: The Study of Aldesleukin with and without antiretroviral therapy (STALWART) evaluated whether intermittent interleukin-2 (IL-2) alone or with antiretroviral therapy (ART) around IL-2 cycles increased CD4(+) counts compared to no therapy.
Methodology: Participants not on continuous ART with > or = 300 CD4(+) cells/mm(3) were randomized to: no treatment; IL-2 for 5 consecutive days every 8 weeks for 3 cycles; or the same IL-2 regimen with 10 days of ART administered around each IL-2 cycle. CD4(+) counts, HIV RNA, and HIV progression events were collected monthly.
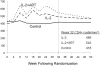
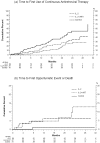
Principal findings: A total of 267 participants were randomized. At week 32, the mean CD4(+) count was 134 cells greater in the IL-2 alone group (p<0.001), and 133 cells greater in the IL-2 plus ART group (p<0.001) compared to the no therapy group. Twelve participants in the IL-2 groups compared to 1 participant in the group assigned to no therapy experienced an opportunistic event or died (HR 5.84, CI: 0.59 to 43.57; p = 0.009).
Conclusions: IL-2 alone or with peri-cycle HAART increases CD4(+) counts but was associated with a greater number of opportunistic events or deaths compared to no therapy. These results call into question the immunoprotective significance of IL-2-induced CD4(+) cells.
Trial registration: ClinicalTrials.gov NCT00110812.
Conflict of interest statement
Figures
Similar articles
-
Comparative gender analysis of the efficacy and safety of atazanavir/ritonavir and lopinavir/ritonavir at 96 weeks in the CASTLE study.J Antimicrob Chemother. 2011 Feb;66(2):363-70. doi: 10.1093/jac/dkq457. Epub 2010 Dec 9. J Antimicrob Chemother. 2011. PMID: 21148235 Free PMC article. Clinical Trial.
-
Lipid-lowering effect and efficacy after switching to etravirine in HIV-infected patients with intolerance to suppressive HAART.HIV Clin Trials. 2013 Jan-Feb;14(1):1-9. doi: 10.1310/hct1401-1. HIV Clin Trials. 2013. PMID: 23372109
-
Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study.Lancet. 2008 Aug 23;372(9639):646-55. doi: 10.1016/S0140-6736(08)61081-8. Lancet. 2008. PMID: 18722869 Clinical Trial.
-
An update and review of antiretroviral therapy.Pharmacotherapy. 2006 Aug;26(8):1111-33. doi: 10.1592/phco.26.8.1111. Pharmacotherapy. 2006. PMID: 16863488 Review.
-
Amprenavir or fosamprenavir plus ritonavir in HIV infection: pharmacology, efficacy and tolerability profile.Drugs. 2005;65(5):633-59. doi: 10.2165/00003495-200565050-00005. Drugs. 2005. PMID: 15748098 Review.
Cited by
-
Gene array studies in HIV-1 infection.Curr HIV/AIDS Rep. 2012 Mar;9(1):34-43. doi: 10.1007/s11904-011-0100-x. Curr HIV/AIDS Rep. 2012. PMID: 22184032 Free PMC article. Review.
-
Involvement of consumers in studies run by the Medical Research Council Clinical Trials Unit: results of a survey.Trials. 2012 Jan 13;13:9. doi: 10.1186/1745-6215-13-9. Trials. 2012. PMID: 22243649 Free PMC article.
-
Role of interleukin-2 in patients with HIV infection.Drugs. 2010 Jun 18;70(9):1115-30. doi: 10.2165/10898620-000000000-00000. Drugs. 2010. PMID: 20518579 Review.
-
The Continuing Evolution of HIV-1 Therapy: Identification and Development of Novel Antiretroviral Agents Targeting Viral and Cellular Targets.Mol Biol Int. 2012;2012:401965. doi: 10.1155/2012/401965. Epub 2012 Jul 10. Mol Biol Int. 2012. PMID: 22848825 Free PMC article.
-
Long-term effects of intermittent IL-2 in HIV infection: extended follow-up of the INSIGHT STALWART Study.PLoS One. 2012;7(10):e47506. doi: 10.1371/journal.pone.0047506. Epub 2012 Oct 17. PLoS One. 2012. PMID: 23082173 Free PMC article. Clinical Trial.
References
-
- Vittinghoffer E, Scheer S, O'Malley P, Colfax G, Holmberg SD, et al. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179:717–720. - PubMed
-
- Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, et al. Changing patterns of mortality across Europe in patients infected with HIV-1 (1998) The EuroSIDA Study Group. Lancet. 1998;352:1725–1730. - PubMed
-
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. - PubMed
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous