A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models
- PMID: 20186321
- PMCID: PMC2826398
- DOI: 10.1371/journal.pone.0009349
A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models
Abstract
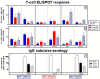
The recent emergence and rapid spread of a novel swine-derived H1N1 influenza virus has resulted in the first influenza pandemic of this century. Monovalent vaccines have undergone preclinical and clinical development prior to initiation of mass immunization campaigns. We have carried out a series of immunogenicity and protection studies following active immunization of mice, which indicate that a whole virus, nonadjuvanted vaccine is immunogenic at low doses and protects against live virus challenge. The immunogenicity in this model was comparable to that of a whole virus H5N1 vaccine, which had previously been demonstrated to induce high levels of seroprotection in clinical studies. The efficacy of the H1N1 pandemic vaccine in protecting against live virus challenge was also seen to be equivalent to that of the H5N1 vaccine. The protective efficacy of the H1N1 vaccine was also confirmed using a severe combined immunodeficient (SCID) mouse model. It was demonstrated that mouse and guinea pig immune sera elicited following active H1N1 vaccination resulted in 100% protection of SCID mice following passive transfer of immune sera and lethal challenge. The immune responses to a whole virus pandemic H1N1 and a split seasonal H1N1 vaccine were also compared in this study. It was demonstrated that the whole virus vaccine induced a balanced Th-1 and Th-2 response in mice, whereas the split vaccine induced mainly a Th-2 response and only minimal levels of Th-1 responses. These data supported the initiation of clinical studies with the same low doses of whole virus vaccine that had previously been demonstrated to be immunogenic in clinical studies with a whole virus H5N1 vaccine.
Conflict of interest statement
Figures


References
-
- Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–470. - PubMed
-
- WHO Pandemic (H1N1) 2009 - update 85. 2010 Available: http://www.who.int/csr/don/2010_01_29/en/index.html. Accessed 8 February 2010.
-
- CDC Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–524. - PubMed
-
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

