Non-invasive detection of a small number of bioluminescent cancer cells in vivo
- PMID: 20186331
- PMCID: PMC2826408
- DOI: 10.1371/journal.pone.0009364
Non-invasive detection of a small number of bioluminescent cancer cells in vivo
Abstract
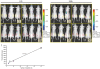
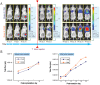
Early detection of tumors can significantly improve the outcome of tumor treatment. One of the most frequently asked questions in cancer imaging is how many cells can be detected non-invasively in a live animal. Although many factors limit such detection, increasing the light emission from cells is one of the most effective ways of overcoming these limitations. Here, we describe development and utilization of a lentiviral vector containing enhanced firefly luciferase (luc2) gene. The resulting single cell clones of the mouse mammary gland tumor (4T1-luc2) showed stable light emission in the range of 10,000 photons/sec/cell. In some cases individual 4T1-luc2 cells inserted under the skin of a nu/nu mouse could be detected non-invasively using a cooled CCD camera in some cases. In addition, we showed that only few cells are needed to develop tumors in these mice and tumor progression can be monitored right after the cells are implanted. Significantly higher luciferase activity in these cells allowed us to detect micrometastases in both, syngeneic Balb/c and nu/nu mice.
Conflict of interest statement
Figures



References
-
- Jenkins DE, Oei Y, Hornig YS, Yu SF, Dusich J, et al. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis. 2003;20:733–744. - PubMed
-
- Hirsch FR, Franklin WA, Gazdar AF, Bunn PA., Jr Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001;7:5–22. - PubMed
-
- Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. - PubMed
-
- Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998;17:279–284. - PubMed
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

