Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1
- PMID: 20186336
- PMCID: PMC2826413
- DOI: 10.1371/journal.pone.0009367
Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1
Abstract
Background: Mitochondrial dysfunction and degradation takes a central role in current paradigms of neurodegeneration in Parkinson's disease (PD). Loss of DJ-1 function is a rare cause of familial PD. Although a critical role of DJ-1 in oxidative stress response and mitochondrial function has been recognized, the effects on mitochondrial dynamics and downstream consequences remain to be determined.
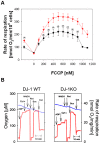
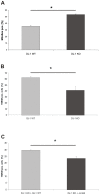
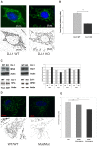
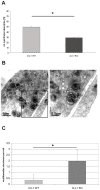
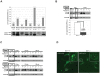
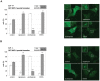
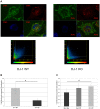
Methodology/principal findings: Using DJ-1 loss of function cellular models from knockout (KO) mice and human carriers of the E64D mutation in the DJ-1 gene we define a novel role of DJ-1 in the integrity of both cellular organelles, mitochondria and lysosomes. We show that loss of DJ-1 caused impaired mitochondrial respiration, increased intramitochondrial reactive oxygen species, reduced mitochondrial membrane potential and characteristic alterations of mitochondrial shape as shown by quantitative morphology. Importantly, ultrastructural imaging and subsequent detailed lysosomal activity analyses revealed reduced basal autophagic degradation and the accumulation of defective mitochondria in DJ-1 KO cells, that was linked with decreased levels of phospho-activated ERK2.
Conclusions/significance: We show that loss of DJ-1 leads to impaired autophagy and accumulation of dysfunctional mitochondria that under physiological conditions would be compensated via lysosomal clearance. Our study provides evidence for a critical role of DJ-1 in mitochondrial homeostasis by connecting basal autophagy and mitochondrial integrity in Parkinson's disease.
Conflict of interest statement
Figures

Similar articles
-
Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics.Hum Mol Genet. 2010 Oct 1;19(19):3734-46. doi: 10.1093/hmg/ddq288. Epub 2010 Jul 16. Hum Mol Genet. 2010. PMID: 20639397
-
Parkinson's disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction.J Neurochem. 2012 Jun;121(5):830-9. doi: 10.1111/j.1471-4159.2012.07734.x. Epub 2012 Apr 12. J Neurochem. 2012. PMID: 22428580 Free PMC article.
-
Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening.PLoS One. 2012;7(7):e40501. doi: 10.1371/journal.pone.0040501. Epub 2012 Jul 9. PLoS One. 2012. PMID: 22792356 Free PMC article.
-
Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson's disease.Curr Opin Neurobiol. 2007 Jun;17(3):331-7. doi: 10.1016/j.conb.2007.04.010. Epub 2007 May 11. Curr Opin Neurobiol. 2007. PMID: 17499497 Review.
-
Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease.Exp Neurol. 2009 Aug;218(2):235-46. doi: 10.1016/j.expneurol.2009.03.006. Epub 2009 Mar 18. Exp Neurol. 2009. PMID: 19303005 Review.
Cited by
-
Role of DJ-1 in Immune and Inflammatory Diseases.Front Immunol. 2020 Jun 16;11:994. doi: 10.3389/fimmu.2020.00994. eCollection 2020. Front Immunol. 2020. PMID: 32612601 Free PMC article. Review.
-
Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway.Anesth Analg. 2013 Apr;116(4):869-80. doi: 10.1213/ANE.0b013e3182860fc9. Epub 2013 Mar 4. Anesth Analg. 2013. PMID: 23460563 Free PMC article.
-
Defective autophagy in Parkinson's disease: role of oxidative stress.Mol Neurobiol. 2012 Dec;46(3):639-61. doi: 10.1007/s12035-012-8318-1. Epub 2012 Aug 17. Mol Neurobiol. 2012. PMID: 22899187 Review.
-
Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4.Free Radic Biol Med. 2013 Dec;65:419-427. doi: 10.1016/j.freeradbiomed.2013.06.030. Epub 2013 Jun 28. Free Radic Biol Med. 2013. PMID: 23816523 Free PMC article.
-
Mitophagy in Parkinson's Disease: From Pathogenesis to Treatment.Cells. 2019 Jul 12;8(7):712. doi: 10.3390/cells8070712. Cells. 2019. PMID: 31336937 Free PMC article. Review.
References
-
- Schiesling C, Kieper N, Seidel K, Kruger R. Review: Familial Parkinson's disease–genetics, clinical phenotype and neuropathology in relation to the common sporadic form of the disease. Neuropathol Appl Neurobiol. 2008;34:255–271. - PubMed
-
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
-
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, et al. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet. 2005;14:2099–2111. - PubMed
-
- Schapira AH. Mitochondrial dysfunction in neurodegenerative diseases. Neurochem Res. 2008;33:2502–2509. - PubMed
-
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, et al. DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol Sci. 2003;24:159–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

