Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress
- PMID: 20186387
- PMCID: PMC2850524
- DOI: 10.1007/s00125-010-1677-0
Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress
Abstract
Aims/hypothesis: Impaired nitric oxide (NO)-dependent vasorelaxation plays a key role in the development of diabetic vascular complications. We investigated the effect of hyperglycaemia on impaired vasoreactivity and a putative role therein of the AGE precursor methylglyoxal.
Methods: The effects of high glucose and methylglyoxal on NO-dependent vasorelaxation in isolated rat mesenteric arteries from wild-type and transgenic glyoxalase (GLO)-I (also known as GLO1) rats, i.e. the enzyme detoxifying methylglyoxal, were recorded in a wire myograph. AGE formation of the major methylglyoxal-adduct 5-hydro-5-methylimidazolone (MG-H1) was detected with an antibody against MG-H1 and quantified with ultra-performance liquid chromatography (tandem) mass spectrometry. Reactive oxygen species formation was measured with a 5-(and-6)-chloromethyl-2'7'-dichlorodihydrofluorescein diacetate acetyl ester probe and by immunohistochemistry with an antibody against nitrotyrosine.
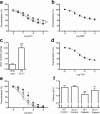
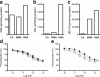
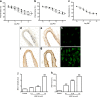
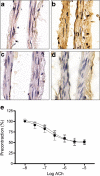
Results: High glucose and methylglyoxal exposure of mesenteric arteries significantly reduced the efficacy of NO-dependent vasorelaxation (p < 0.05). This impairment was not observed in mesenteric arteries of GLO-I transgenic rats indicating a specific intracellular methylglyoxal effect. The diabetes-induced impaired potency (pD(2)) in mesenteric arteries of wild-type rats was significantly improved by GLO-I overexpression (p < 0.05). Methylglyoxal-modified albumin did not affect NO-dependent vasorelaxation, while under the same conditions the receptor for AGE ligand S100b did (p < 0.05). Methylglyoxal treatment of arteries increased intracellular staining of MG-H1 in endothelial cells and adventitia by fivefold accompanied by an eightfold increase in the oxidative stress marker nitrotyrosine. Antioxidant pre-incubation prevented methylglyoxal-induced impairment of vasoreactivity.
Conclusions/interpretation: These data show that hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation is mediated by increased intracellular methylglyoxal levels in a pathway dependent on oxidative stress.
Figures
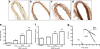
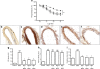
References
-
- Makimattila S, Virkamaki A, Groop PH, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. - PubMed
-
- Diederich D, Skopec J, Diederich A, Dai FX. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am J Physiol. 1994;266:H1153–H1161. - PubMed
-
- Pflueger AC, Osswald H, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of nitric oxide. Am J Physiol. 1999;276:F340–F346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

