The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels
- PMID: 20186732
- PMCID: PMC2888622
- DOI: 10.1002/jbm.a.32704
The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels
Abstract
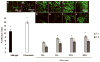
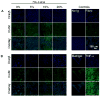
In recent studies, we showed that exogenous hyaluronic acid oligomers (HA-o) stimulate functional endothelialization, though native long-chain HA is more bioinert and possibly more biocompatible. Thus, in this study, hydrogels containing high molecular weight (HMW) HA (1 x 10(6) Da) and HA-o mixtures (HA-o: 0.75-10 kDa) were created by crosslinking with divinyl sulfone (DVS). The incorporation of HA-o was found to compromise the physical and mechanical properties of the gels (rheology, apparent crosslinking density, swelling ratio, degradation) and to very mildly enhance inflammatory cell recruitment in vivo; increasing the DVS crosslinker content within the gels in general, had the opposite effect, though the relatively high concentration of DVS within these gels (necessary to create a solid gel) also stimulated a mild subcutaneous inflammatory response in vivo and VCAM-1 expression by endothelial cells (ECs) cultured atop; ICAM-expression levels remained very low irrespective extent of DVS crosslinking or HA-o content. The greatest EC attachment and proliferation (MTT assay) was observed on gels that contained the highest amount of HA-o. The study shows that the beneficial EC response to HA-o and biocompatibility of HA is mostly unaltered by their chemical derivatization and crosslinking into a hydrogel. However, the study also demonstrates that the relatively high concentrations of DVS, necessary to create solid gels, compromise their biocompatibility. Moreover, the poor mechanics of even these heavily crosslinked gels, in the context of vascular implantation, necessitates the investigation of other, more appropriate crosslinking agents. Alternately, the outcomes of this study may be used to guide an approach based on chemical immobilization and controlled surface-presentation of both bioactive HA-o and more biocompatible HMW HA on synthetic or tissue engineered grafts already in use, without the use of a crosslinker, so that improved, predictable, and functional endothelialization can be achieved, and the need to create a mechanically compliant biomaterial for standalone use, circumvented.
(c) 2010 Wiley Periodicals, Inc.
Figures









Similar articles
-
Characterization of glycidyl methacrylate - crosslinked hyaluronan hydrogel scaffolds incorporating elastogenic hyaluronan oligomers.Acta Biomater. 2011 Feb;7(2):653-65. doi: 10.1016/j.actbio.2010.08.006. Epub 2010 Aug 13. Acta Biomater. 2011. PMID: 20709199 Free PMC article.
-
Physical properties of crosslinked hyaluronic acid hydrogels.J Mater Sci Mater Med. 2008 Nov;19(11):3335-43. doi: 10.1007/s10856-008-3476-4. Epub 2008 Jun 5. J Mater Sci Mater Med. 2008. PMID: 18528637
-
Anti-inflammatory drug delivery from hyaluronic acid hydrogels.J Biomater Sci Polym Ed. 2004;15(9):1111-9. doi: 10.1163/1568562041753115. J Biomater Sci Polym Ed. 2004. PMID: 15503629
-
Dynamic covalent crosslinked hyaluronic acid hydrogels and nanomaterials for biomedical applications.Biomater Sci. 2022 Nov 8;10(22):6399-6412. doi: 10.1039/d2bm01154a. Biomater Sci. 2022. PMID: 36214100 Review.
-
Advances in modified hyaluronic acid-based hydrogels for skin wound healing.Biomater Sci. 2022 Jun 28;10(13):3393-3409. doi: 10.1039/d2bm00397j. Biomater Sci. 2022. PMID: 35575243 Review.
Cited by
-
Investigation of the degradation-retarding effect caused by the low swelling capacity of a novel hyaluronic Acid filler developed by solid-phase crosslinking technology.Ann Dermatol. 2014 Jun;26(3):357-62. doi: 10.5021/ad.2014.26.3.357. Epub 2014 Jun 12. Ann Dermatol. 2014. PMID: 24966636 Free PMC article.
-
Crosslinking method of hyaluronic-based hydrogel for biomedical applications.J Tissue Eng. 2017 Sep 6;8:2041731417726464. doi: 10.1177/2041731417726464. eCollection 2017 Jan-Dec. J Tissue Eng. 2017. PMID: 28912946 Free PMC article.
-
Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery.Pharmaceutics. 2019 Aug 12;11(8):407. doi: 10.3390/pharmaceutics11080407. Pharmaceutics. 2019. PMID: 31408954 Free PMC article. Review.
-
Interpenetrating Low-Molecular Weight Hyaluronic Acid in Hyaluronic Acid-Based In Situ Hydrogel Scaffold for Periodontal and Oral Wound Applications.Polymers (Basel). 2022 Nov 17;14(22):4986. doi: 10.3390/polym14224986. Polymers (Basel). 2022. PMID: 36433112 Free PMC article.
-
Nanocomposite hyaluronic acid-based hydrogel for the treatment of esophageal fistulas.Mater Today Bio. 2021 Mar 27;10:100109. doi: 10.1016/j.mtbio.2021.100109. eCollection 2021 Mar. Mater Today Bio. 2021. PMID: 33997760 Free PMC article.
References
-
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–64. - PubMed
-
- Tan SW, Johns MR, Greenfield PF. Hyaluronic acid--a versatile biopolymer. Aust J Biotechnol. 1990;4(1):38–43. - PubMed
-
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. - PubMed
-
- Morra M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules. 2005;6(3):1205–23. - PubMed
-
- Wei YT, Tian WM, Yu X, Cui FZ, Hou SP, Xu QY, Lee IS. Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed Mater. 2007;2(3):S142–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

