Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells
- PMID: 20186733
- PMCID: PMC2891839
- DOI: 10.1002/jbm.a.32696
Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells
Abstract
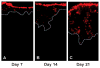
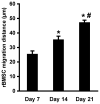
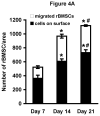
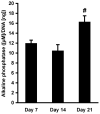
Hydrogels are potentially useful for many purposes in regenerative medicine including drug and growth factor delivery, as single scaffold for bone repair or as a filler of pores of another biomaterial in which host mesenchymal progenitor cells can migrate in and differentiate into matrix-producing osteoblasts. Collagen type I is of special interest as it is a very important and abundant natural matrix component. The purpose of this study was to investigate whether rat bone marrow stromal cells (rBMSCs) are able to adhere to, to survive, to proliferate and to migrate in collagen type I hydrogels and whether they can adopt an osteoblastic fate. rBMSCs were obtained from rat femora and plated on collagen type I hydrogels. Before harvest by day 7, 14, and 21, hydrogels were fluorescently labeled, cryo-cut and analyzed by fluorescent-based and laser scanning confocal microscopy to determine cell proliferation, migration, and viability. Osteogenic differentiation was determined by alkaline phosphatase activity. Collagen type I hydrogels allowed the attachment of rBMSCs to the hydrogel, their proliferation, and migration towards the inner part of the gel. rBMSCs started to differentiate into osteoblasts as determined by an increase in alkaline phosphatase activity after two weeks in culture. This study therefore suggests that collagen type I hydrogels could be useful for musculoskeletal regenerative therapies.
(c) 2010 Wiley Periodicals, Inc.
Figures



Similar articles
-
[Effect of Biaxial Tensile Strain on Expression of Osteogenic Specificity Markers of Rat Bone Marrow-derived Mesenchymal Stem Cells in Vitro].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2016 Jun;33(3):499-505. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2016. PMID: 29709150 Chinese.
-
Effect of hydrogel porosity on marrow stromal cell phenotypic expression.Biomaterials. 2008 May;29(14):2193-202. doi: 10.1016/j.biomaterials.2008.01.006. Epub 2008 Feb 11. Biomaterials. 2008. PMID: 18262642 Free PMC article.
-
Osteogenic differentiation of rat bone marrow stromal cells cultured on Arg-Gly-Asp modified hydrogels without dexamethasone and beta-glycerol phosphate.Biomaterials. 2005 Jun;26(17):3645-54. doi: 10.1016/j.biomaterials.2004.09.050. Biomaterials. 2005. PMID: 15621255
-
Biomimetic collagen scaffolds for human bone cell growth and differentiation.Tissue Eng. 2004 Jul-Aug;10(7-8):1148-59. doi: 10.1089/ten.2004.10.1148. Tissue Eng. 2004. PMID: 15363171
-
Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels.J Biomed Mater Res A. 2003 Sep 1;66(3):698-706. doi: 10.1002/jbm.a.10003. J Biomed Mater Res A. 2003. PMID: 12918054
Cited by
-
Matrices Activated with Messenger RNA.J Funct Biomater. 2023 Jan 15;14(1):48. doi: 10.3390/jfb14010048. J Funct Biomater. 2023. PMID: 36662095 Free PMC article. Review.
-
Matrix-bound Cyr61/CCN1 is required to retain the properties of the bone marrow mesenchymal stem cell niche but is depleted with aging.Matrix Biol. 2022 Aug;111:108-132. doi: 10.1016/j.matbio.2022.06.004. Epub 2022 Jun 23. Matrix Biol. 2022. PMID: 35752272 Free PMC article.
-
Tissue specific stem cell therapy for airway regeneration.Cell Prolif. 2024 Oct;57(10):e13662. doi: 10.1111/cpr.13662. Epub 2024 May 27. Cell Prolif. 2024. PMID: 38803033 Free PMC article.
-
Biomedical Applications of Biodegradable Polymers.J Polym Sci B Polym Phys. 2011 Jun 15;49(12):832-864. doi: 10.1002/polb.22259. J Polym Sci B Polym Phys. 2011. PMID: 21769165 Free PMC article.
-
Hydrogel Composite Incorporating Deferoxamine-Loaded Gelatin-Based Microspheres Enhance Angiogenesis Ability of Dental Pulp Stem Cells.ACS Omega. 2025 Mar 21;10(12):12579-12589. doi: 10.1021/acsomega.5c00445. eCollection 2025 Apr 1. ACS Omega. 2025. PMID: 40191326 Free PMC article.
References
-
- Bikram M, Fouletier-Dilling C, Hipp JA, Gannon F, Davis AR, Olmsted-Davis EA, West JL. Endochondral bone formation from hydrogel carriers loaded with BMP2-transduced cells. Ann Biomed Eng. 2007;35:796–807. - PubMed
-
- Giannoni P, Mastrogiacomo M, Alini M, Pearce SG, Corsi A, Santolini F, Muraglia A, Bianco P, Cancedda R. Regeneration of large bone defects in sheep using bone marrow stromal cells. J Tissue Eng Regen Med. 2008;2:253–262. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

