APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging
- PMID: 20186853
- PMCID: PMC2830375
- DOI: 10.1002/ana.21843
APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging
Abstract
Objective: To examine interactions of apolipoprotein E (APOE) genotype with age and with in vivo measures of preclinical Alzheimer disease (AD) in cognitively normal aging.
Methods: Two hundred forty-one cognitively normal individuals, aged 45-88 years, had cerebral amyloid imaging studies with Pittsburgh Compound-B (PIB). Of the 241 individuals, 168 (70%) also had cerebrospinal fluid (CSF) assays of amyloid-beta(42) (Abeta(42)), tau, and phosphorylated tau (ptau(181)). All individuals were genotyped for APOE.
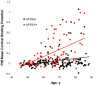
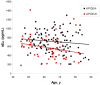
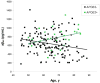
Results: The frequency of individuals with elevated mean cortical binding potential (MCBP) for PIB rose in an age-dependent manner from 0% at ages 45-49 years to 30.3% at 80-88 years. Reduced levels of CSF Abeta(42) appeared to begin earlier (18.2% of those aged 45-49 years) and increase with age in higher frequencies (50% at age 80-88 years) than elevations of MCBP. There was a gene dose effect for the APOE4 genotype, with greater MCBP increases and greater reductions in CSF Abeta(42) with increased numbers of APOE4 alleles. Individuals with an APOE2 allele had no increase in MCBP with age and had higher CSF Abeta(42) levels than individuals without an APOE2 allele. There was no APOE4 or APOE2 effect on CSF tau or ptau(181).
Interpretation: Increasing cerebral Abeta deposition with age is the pathobiological phenotype of APOE4. The biomarker sequence that detects Abeta deposition may first be lowered CSF Abeta(42), followed by elevated MCBP for PIB. A substantial proportion of cognitively normal individuals have presumptive preclinical AD.
Figures





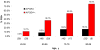
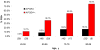
References
-
- Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. - PubMed
-
- Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. - PubMed
-
- Troncoso JC, Cataldo M, Nixon RA, et al. Neuropathology of preclinical and clinical late-onset Alzheimer’s disease. Ann Neurol. 1998;43:673–676. - PubMed
-
- Hulette CM, Welsh-Bohmer KA, Murray MG, et al. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. - PubMed
-
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

