Distinctive ERK and p38 signaling in remote and infarcted myocardium during post-MI remodeling in the mouse
- PMID: 20186881
- PMCID: PMC3397305
- DOI: 10.1002/jcb.22498
Distinctive ERK and p38 signaling in remote and infarcted myocardium during post-MI remodeling in the mouse
Abstract
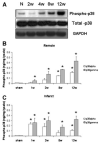
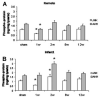
Global activation of MAP kinases has been reported in both human and experimental heart failure. Chronic remodeling of the surviving ventricular wall after myocardial infarction (MI) involves both myocyte loss and fibrosis; we hypothesized that this cardiomyopathy involves differential shifts in pro- and anti-apoptotic MAP kinase signaling in cardiac myocyte (CM) and non-myocyte. Cardiomyopathy after coronary artery ligation in mice was characterized by echocardiography, ex vivo Langendorff preparation, histologic analysis and measurements of apoptosis. Phosphorylation (activation) of signaling molecules was analyzed by Western blot, ELISA and immunohistochemistry. Post-MI remodeling involved dramatic changes in the phosphorylation of both stress-activated MAP (SAP) kinase p38 as well as ERK, a known mediator of cell survival, but not of SAP kinase JNK or the anti-apoptotic mediator of PI3K, Akt. Phosphorylation of p38 rose early after MI in the infarct, whereas a more gradual rise in the remote myocardium accompanied a rise in apoptosis in that region. In both areas, ERK phosphorylation was lowest early after MI and rose steadily thereafter, though infarct phosphorylation was consistently higher. Immunostaining of p-ERK localized to fibrotic areas populated primarily by non-myocytes, whereas staining of p38 phosphorylation was stronger in areas of progressive CM apoptosis. Relative segregation of CMs and non-myocytes in different regions of the post-MI myocardium revealed signaling patterns that imply cell type-specific changes in pro- and anti-apoptotic MAP kinase signaling. Prevention of myocyte loss and of LV remodeling after MI may therefore require cell type-specific manipulation of p38 and ERK activation.
Figures



Similar articles
-
Progressive left ventricular remodeling, myocyte apoptosis, and protein signaling cascades after myocardial infarction in rabbits.Biochim Biophys Acta. 2005 Jun 10;1740(3):499-513. doi: 10.1016/j.bbadis.2004.11.007. Epub 2004 Dec 8. Biochim Biophys Acta. 2005. PMID: 15949720
-
p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat.J Am Coll Cardiol. 2004 Oct 19;44(8):1679-89. doi: 10.1016/j.jacc.2004.07.038. J Am Coll Cardiol. 2004. PMID: 15489104
-
Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats.Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H709-15. doi: 10.1152/ajpheart.00186.2005. Epub 2005 Sep 23. Am J Physiol Heart Circ Physiol. 2006. PMID: 16183734
-
Ataxia telangiectasia-mutated kinase deficiency exacerbates left ventricular dysfunction and remodeling late after myocardial infarction.Am J Physiol Heart Circ Physiol. 2016 Aug 1;311(2):H445-52. doi: 10.1152/ajpheart.00338.2016. Epub 2016 Jun 10. Am J Physiol Heart Circ Physiol. 2016. PMID: 27288435 Free PMC article.
-
Signaling Pathways and Potential Therapeutic Strategies in Cardiac Fibrosis.Int J Mol Sci. 2023 Jan 16;24(2):1756. doi: 10.3390/ijms24021756. Int J Mol Sci. 2023. PMID: 36675283 Free PMC article. Review.
Cited by
-
miR-212 Promotes Cardiomyocyte Hypertrophy through Regulating Transcription Factor 7 Like 2.Mediators Inflamm. 2022 Aug 24;2022:5187218. doi: 10.1155/2022/5187218. eCollection 2022. Mediators Inflamm. 2022. PMID: 36060928 Free PMC article.
-
Vascular endothelial growth factor-C: its unrevealed role in fibrogenesis.Am J Physiol Heart Circ Physiol. 2014 Mar;306(6):H789-96. doi: 10.1152/ajpheart.00559.2013. Epub 2014 Jan 24. Am J Physiol Heart Circ Physiol. 2014. PMID: 24464750 Free PMC article.
-
The Stress-Response MAP Kinase Signaling in Cardiac Arrhythmias.Rev Physiol Biochem Pharmacol. 2016;172:77-100. doi: 10.1007/112_2016_8. Rev Physiol Biochem Pharmacol. 2016. PMID: 27848025 Free PMC article. Review.
-
Shift toward greater pathologic post-myocardial infarction remodeling with loss of the adaptive hypertrophic signaling of alpha1 adrenergic receptors in mice.PLoS One. 2017 Dec 7;12(12):e0188471. doi: 10.1371/journal.pone.0188471. eCollection 2017. PLoS One. 2017. PMID: 29216197 Free PMC article.
-
Asiatic acid inhibits left ventricular remodeling and improves cardiac function in a rat model of myocardial infarction.Exp Ther Med. 2016 Jan;11(1):57-64. doi: 10.3892/etm.2015.2871. Epub 2015 Nov 17. Exp Ther Med. 2016. PMID: 26889217 Free PMC article.
References
-
- Abbate A, Bussani R, Biondi-Zoccai GG, Rossiello R, Silvestri F, Baldi F, Biasucci LM, Baldi A. Persistent infarct-related artery occlusion is associated with an increased myocardial apoptosis at postmortem examination in humans late after an acute myocardial infarction. Circulation. 2002;106:1051–1054. - PubMed
-
- Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, Di Sciascio G. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol. 2002;34:165–174. - PubMed
-
- Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell Death Differ. 2007;14:1060–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

