Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids
- PMID: 20187040
- PMCID: PMC3786736
- DOI: 10.1002/chem.200902880
Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids
Abstract
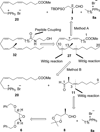
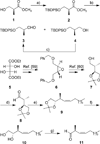
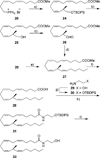
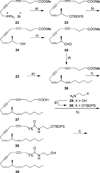
Two novel methyl-substituted arachidonic acid derivatives were prepared in an enantioselective manner from commercially available chiral building blocks, and were found to be excellent templates for the development of (13S)-methyl-substituted anandamide analogues. One of the compounds synthesized, namely, (13S,5Z,8Z,11Z,14Z)-13-methyl-eicosa-5,8,11,14-tetraenoic acid N-(2-hydroxyethyl)amide, is an endocannabinoid analogue with remarkably high affinity for the CB1 cannabinoid receptor.
Figures


Similar articles
-
Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase.J Med Chem. 2002 Aug 15;45(17):3709-20. doi: 10.1021/jm020818q. J Med Chem. 2002. PMID: 12166944
-
High affinity electrophilic and photoactivatable covalent endocannabinoid probes for the CB1 receptor.J Med Chem. 2005 Oct 6;48(20):6423-9. doi: 10.1021/jm050272i. J Med Chem. 2005. PMID: 16190768
-
Synthesis, conformational analysis and CB1 binding affinity of hairpin-like anandamide pseudopeptide mimetics.J Pept Sci. 2006 Sep;12(9):575-91. doi: 10.1002/psc.754. J Pept Sci. 2006. PMID: 16534762
-
Oleamide: a member of the endocannabinoid family?Br J Pharmacol. 2004 Jan;141(2):195-6. doi: 10.1038/sj.bjp.0705608. Epub 2003 Dec 22. Br J Pharmacol. 2004. PMID: 14691053 Free PMC article. Review.
-
Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown.Curr Med Chem. 2010;17(14):1468-86. doi: 10.2174/092986710790980005. Curr Med Chem. 2010. PMID: 20166921 Free PMC article. Review.
Cited by
-
Enantioselective synthesis of (10S)- and (10R)-methyl-anandamides.Tetrahedron. 2012 Aug 5;68(31):10.1016/j.tet.2012.05.010. doi: 10.1016/j.tet.2012.05.010. Tetrahedron. 2012. PMID: 24319298 Free PMC article.
-
C-ring cannabinoid lactones: a novel cannabinergic chemotype.ACS Med Chem Lett. 2014 Jan 14;5(4):400-4. doi: 10.1021/ml4005304. eCollection 2014 Apr 10. ACS Med Chem Lett. 2014. PMID: 24900848 Free PMC article.
-
Novel tail and head group prostamide probes.Bioorg Med Chem Lett. 2015 Mar 15;25(6):1228-31. doi: 10.1016/j.bmcl.2015.01.064. Epub 2015 Feb 4. Bioorg Med Chem Lett. 2015. PMID: 25701254 Free PMC article.
-
Chiral Me-2-arachidonoyl Glycerols: The First Potent Endocannabinoid Glyceride Templates with Stability to COX-2.ACS Med Chem Lett. 2024 May 28;15(6):965-971. doi: 10.1021/acsmedchemlett.4c00175. eCollection 2024 Jun 13. ACS Med Chem Lett. 2024. PMID: 38894922 Free PMC article.
-
Terpenes and lipids of the endocannabinoid and transient-receptor-potential-channel biosignaling systems.ACS Chem Neurosci. 2014 Nov 19;5(11):1097-106. doi: 10.1021/cn5000875. Epub 2014 Jun 5. ACS Chem Neurosci. 2014. PMID: 24866555 Free PMC article. Review.
References
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. - PubMed
-
- T Sugiura, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem. Biophys. Res. Commun. 1995;215:89–97. - PubMed
-
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem. Pharmacol. 1995;50:83–90. - PubMed
-
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Mol. Pharmacol. 1988;34:605–613. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

