Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids
- PMID: 20187040
- PMCID: PMC3786736
- DOI: 10.1002/chem.200902880
Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids
Abstract
Two novel methyl-substituted arachidonic acid derivatives were prepared in an enantioselective manner from commercially available chiral building blocks, and were found to be excellent templates for the development of (13S)-methyl-substituted anandamide analogues. One of the compounds synthesized, namely, (13S,5Z,8Z,11Z,14Z)-13-methyl-eicosa-5,8,11,14-tetraenoic acid N-(2-hydroxyethyl)amide, is an endocannabinoid analogue with remarkably high affinity for the CB1 cannabinoid receptor.
Figures
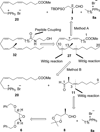
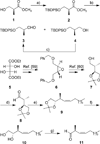


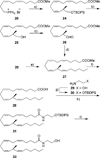
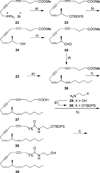
References
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. - PubMed
-
- T Sugiura, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. Biochem. Biophys. Res. Commun. 1995;215:89–97. - PubMed
-
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Biochem. Pharmacol. 1995;50:83–90. - PubMed
-
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Mol. Pharmacol. 1988;34:605–613. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Nature. 1993;365:61–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

