The ubiquitin C-terminal hydrolase UCH-L1 regulates B-cell proliferation and integrin activation
- PMID: 20187292
- PMCID: PMC6512389
- DOI: 10.1111/j.1582-4934.2008.00501.x
The ubiquitin C-terminal hydrolase UCH-L1 regulates B-cell proliferation and integrin activation
Abstract
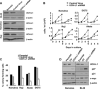
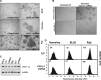
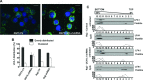
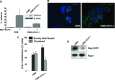
The ubiquitin C-terminal hydrolase-L1 (UCH-L1) is a deubiquitinating enzyme that catalyses the hydrolysis of polyubiquitin precursors and small ubiquitin adducts. UCH-L1 has been detected in a variety of malignant and metastatic tumours but its biological function in these cells is unknown. We have previously shown that UCH-L1 is highly expressed in Burkitt's lymphoma (BL) and is up-regulated upon infection of B lymphocytes with Epstein-Barr virus (EBV). Here we show that knockdown of UCH-L1 by RNAi inhibits the proliferation of BL cells in suspension and semisolid agar and activates strong LFA-1-dependent homotypic adhesion. Induction of cell adhesion correlated with cation-induced binding to ICAM-1, clustering of LFA-1 into lipid rafts and constitutive activation of the Rap1 and Rac1 GTPases. Expression of a catalytically active UCH-L1 promoted the proliferation of a UCH-L1-negative EBV transformed lymphoblastoid cell line (LCL) and inhibited cell adhesion, whereas a catalytic mutant had no effect, confirming the requirement of UCH-L1 enzymatic activity for the regulation of these phenotypes. Our results identify UCH-L1 as a new player in the signalling pathways that promote the proliferation and invasive capacity of malignant B cells.
Figures



Similar articles
-
Inhibition of UCH-L1 Deubiquitinating Activity with Two Forms of LDN-57444 Has Anti-Invasive Effects in Metastatic Carcinoma Cells.Int J Mol Sci. 2019 Jul 31;20(15):3733. doi: 10.3390/ijms20153733. Int J Mol Sci. 2019. PMID: 31370144 Free PMC article.
-
Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes.PLoS One. 2009 Aug 26;4(8):e6764. doi: 10.1371/journal.pone.0006764. PLoS One. 2009. PMID: 19707515 Free PMC article.
-
PU.1-dependent regulation of UCH L1 expression in B-lymphoma cells.Leuk Lymphoma. 2011 Jul;52(7):1336-47. doi: 10.3109/10428194.2011.562571. Epub 2011 Apr 20. Leuk Lymphoma. 2011. PMID: 21504384 Free PMC article.
-
Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction.Biochem J. 2016 Aug 15;473(16):2453-62. doi: 10.1042/BCJ20160082. Biochem J. 2016. PMID: 27515257 Free PMC article. Review.
-
The potential role of ubiquitin c-terminal hydrolases in oncogenesis.Biochim Biophys Acta. 2010 Aug;1806(1):1-6. doi: 10.1016/j.bbcan.2010.03.001. Epub 2010 Mar 17. Biochim Biophys Acta. 2010. PMID: 20302916 Review.
Cited by
-
Deubiquitinases in hematological malignancies.Biomark Res. 2021 Aug 28;9(1):66. doi: 10.1186/s40364-021-00320-w. Biomark Res. 2021. PMID: 34454635 Free PMC article. Review.
-
C-Terminal Farnesylation of UCH-L1 Plays a Role in Transport of Epstein-Barr Virus Primary Oncoprotein LMP1 to Exosomes.mSphere. 2018 Feb 7;3(1):e00030-18. doi: 10.1128/mSphere.00030-18. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29435490 Free PMC article.
-
Ubiquitin C-terminal hydrolase-L1 increases cancer cell invasion by modulating hydrogen peroxide generated via NADPH oxidase 4.Oncotarget. 2015 Jun 30;6(18):16287-303. doi: 10.18632/oncotarget.3843. Oncotarget. 2015. PMID: 25915537 Free PMC article.
-
Inhibition of UCH-L1 Deubiquitinating Activity with Two Forms of LDN-57444 Has Anti-Invasive Effects in Metastatic Carcinoma Cells.Int J Mol Sci. 2019 Jul 31;20(15):3733. doi: 10.3390/ijms20153733. Int J Mol Sci. 2019. PMID: 31370144 Free PMC article.
-
Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/TCF signaling.PLoS One. 2009 Jun 18;4(6):e5955. doi: 10.1371/journal.pone.0005955. PLoS One. 2009. PMID: 19536331 Free PMC article.
References
-
- Ciechanover A, Schwartz AL. The ubiquitin system: pathogenesis of human diseases and drug targeting. Biochim Biophys Acta . 2004; 1695: 3–17. - PubMed
-
- Love KR, Catic A, Schlieker C, et al. Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nat Chem Biol . 2007; 3: 697–705. - PubMed
-
- Nijman SM, Luna‐Vargas MP, Velds A, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell . 2005; 123: 773–86. - PubMed
-
- Masucci MG. Epstein‐Barr virus oncogenesis and the ubiquitin‐proteasome system. Oncogene . 2004; 23: 2107–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

