Developmental tumourigenesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population
- PMID: 20187302
- PMCID: PMC6529968
- DOI: 10.1111/j.1582-4934.2008.00607.x
Developmental tumourigenesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population
Abstract
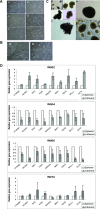
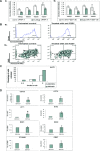
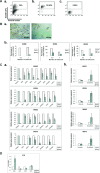
During development, renal stem cells reside in the nephrogenic blastema. Wilms' tumour (WT), a common childhood malignancy, is suggested to arise from the nephrogenic blastema that undergoes partial differentiation and as such is an attractive model to study renal stem cells leading to cancer initiation and maintenance. Previously we have made use of blastema-enriched WT stem-like xenografts propagated in vivo to define a 'WT-stem' signature set, which includes cell surface markers convenient for cell isolation (frizzled homolog 2 [Drosophila] - FZD2, FZD7, G-protein coupled receptor 39, activin receptor type 2B, neural cell adhesion molecule - NCAM). We show by fluorescenceactivated cell sorting analysis of sphere-forming heterogeneous primary WT cultures that most of these markers and other stem cell surface antigens (haematopoietic, CD133, CD34, c-Kit; mesenchymal, CD105, CD90, CD44; cancer, CD133, MDR1; hESC, CD24 and putative renal, cadherin 11), are expressed in WT cell sub-populations in varying levels. Of all markers, NCAM, CD133 and FZD7 were constantly detected in low-to-moderate portions likely to contain the stem cell fraction. Sorting according to FZD7 resulted in extensive cell death, while sorted NCAM and CD133 cell fractions were subjected to clonogenicity assays and quantitative RT-PCR analysis, exclusively demonstrating the NCAM fraction as highly clonogenic, overexpressing the WT 'stemness' genes and topoisomerase2A (TOP2A), a bad prognostic marker for WT. Moreover, treatment of WT cells with the topoisomerase inhibitors, Etoposide and Irinotecan resulted in down-regulation of TOP2A along with NCAM and WT1. Thus, we suggest NCAM as a marker for the WT progenitor cell population. These findings provide novel insights into the cellular hierarchy of WT, having possible implications for future therapeutic options.
Figures




Similar articles
-
Expression of stem cell markers in the human fetal kidney.PLoS One. 2009 Aug 21;4(8):e6709. doi: 10.1371/journal.pone.0006709. PLoS One. 2009. PMID: 19696931 Free PMC article.
-
Resistance or sensitivity of Wilms' tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target.Oncogene. 2011 Apr 7;30(14):1664-80. doi: 10.1038/onc.2010.549. Epub 2011 Jan 17. Oncogene. 2011. PMID: 21472018
-
Blastemal NCAM+ALDH1+ Wilms' tumor cancer stem cells correlate with disease progression and poor clinical outcome: A pilot study.Pathol Res Pract. 2019 Aug;215(8):152491. doi: 10.1016/j.prp.2019.152491. Epub 2019 Jun 10. Pathol Res Pract. 2019. PMID: 31202518
-
Wilms tumor--a renal stem cell malignancy?Pediatr Nephrol. 2011 Sep;26(9):1535-43. doi: 10.1007/s00467-011-1858-1. Epub 2011 Apr 16. Pediatr Nephrol. 2011. PMID: 21499773 Review.
-
Nephroblastoma (Wilms' tumor): a model system of aberrant renal development.Semin Diagn Pathol. 1994 May;11(2):126-35. Semin Diagn Pathol. 1994. PMID: 7809506 Review.
Cited by
-
Molecular subtyping and characterization of clear cell renal cell carcinoma by tumor differentiation trajectories.iScience. 2023 Oct 30;26(12):108370. doi: 10.1016/j.isci.2023.108370. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034348 Free PMC article.
-
Single-Cell Transcriptional Landscape Reveals the Regulatory Network and Its Heterogeneity of Renal Mitochondrial Damages in Diabetic Kidney Disease.Int J Mol Sci. 2023 Aug 31;24(17):13502. doi: 10.3390/ijms241713502. Int J Mol Sci. 2023. PMID: 37686311 Free PMC article.
-
Rapid deterioration of preexisting renal insufficiency after autologous mesenchymal stem cell therapy.Kidney Res Clin Pract. 2017 Jun;36(2):200-204. doi: 10.23876/j.krcp.2017.36.2.200. Epub 2017 Jun 30. Kidney Res Clin Pract. 2017. PMID: 28680828 Free PMC article.
-
Gene Expression Analysis of (Paired) Primary and Relapsed Wilms Tumor Samples to Unravel the Underlying Factors Driving Tumor Recurrence.Cancer Med. 2025 Jun;14(11):e70969. doi: 10.1002/cam4.70969. Cancer Med. 2025. PMID: 40439002 Free PMC article.
-
Chromatin-modifying agents reactivate embryonic renal stem/progenitor genes in human adult kidney epithelial cells but abrogate dedifferentiation and stemness.Cell Reprogram. 2013 Aug;15(4):281-92. doi: 10.1089/cell.2012.0087. Epub 2013 Jul 10. Cell Reprogram. 2013. PMID: 23841748 Free PMC article.
References
-
- Rivera MN, Haber DA. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer. 2005; 5: 699–712. - PubMed
-
- Feinberg AP, Williams BR. Wilms’ tumor as a model for cancer biology. Methods Mol Biol. 2003; 222: 239–48. - PubMed
-
- Dekel B, Metsuyanim S, Schmidt‐Ott KM, et al . Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Res. 2006; 66: 6040–9. - PubMed
-
- Natrajan R, Reis‐Filho JS, Little SE, et al . Blastemal expression of type I insulin‐like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006; 66: 11148–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

