Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach
- PMID: 20187635
- PMCID: PMC2893734
- DOI: 10.1021/np900821e
Discovery and development of natural product-derived chemotherapeutic agents based on a medicinal chemistry approach
Abstract
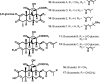
Medicinal plants have long been an excellent source of pharmaceutical agents. Accordingly, the long-term objectives of the author's research program are to discover and design new chemotherapeutic agents based on plant-derived compound leads by using a medicinal chemistry approach, which is a combination of chemistry and biology. Different examples of promising bioactive natural products and their synthetic analogues, including sesquiterpene lactones, quassinoids, naphthoquinones, phenylquinolones, dithiophenediones, neo-tanshinlactone, tylophorine, suksdorfin, DCK, and DCP, will be presented with respect to their discovery and preclinical development as potential clinical trial candidates. Research approaches include bioactivity- or mechanism of action-directed isolation and characterization of active compounds, rational drug design-based modification and analogue synthesis, and structure-activity relationship and mechanism of action studies. Current clinical trial agents discovered by the Natural Products Research Laboratories, University of North Carolina, include bevirimat (dimethyl succinyl betulinic acid), which is now in phase IIb trials for treating AIDS. Bevirimat is also the first in a new class of HIV drug candidates called "maturation inhibitors". In addition, an etoposide analogue, GL-331, progressed to anticancer phase II clinical trials, and the curcumin analogue JC-9 is in phase II clinical trials for treating acne and in development for trials against prostate cancer. The discovery and development of these clinical trial candidates will also be discussed.
Figures


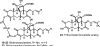
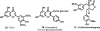
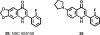
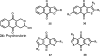
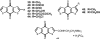




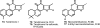
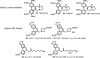



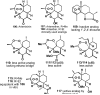
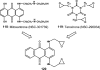
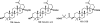

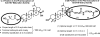
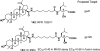
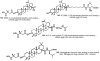
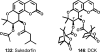
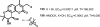
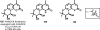


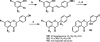

References
-
- Lee KH. Public Health Nutrition. 2000;3:515–522. - PubMed
-
- Lee KH, Furukawa H, Kozuka M, Huang HC, Luhan PA, McPhail AT. J. Chem. Soc., Chem. Commun. 1973:476–477.
-
- Lee KH, Ibuka T, Huang HC, Harris DL. J. Pharm. Sci. 1976;62:1077–1078. - PubMed
-
- McPhail AT, Onan KD, Lee KH, Ibuka T, Kozuka M, Shingu T, Huang HC. Tetrahedron Lett. 1974;32:2739–2741.
-
- Lee KH, Imakura Y, Sims D, McPhail AT, Onan KD. J. Chem. Soc., Chem. Commun. 1976:341–342.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

