Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones
- PMID: 20187640
- PMCID: PMC2840677
- DOI: 10.1021/ja100014q
Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones
Abstract
Bioorthogonal chemical reactions, those that do not interact or interfere with biology, have allowed for exploration of numerous biological processes that were previously difficult to study. The reaction of azides with strained alkynes, such as cyclooctynes, readily forms a triazole product without the need for a toxic catalyst. Here we describe a biarylazacyclooctynone (BARAC) that has exceptional reaction kinetics and whose synthesis is designed to be both modular and scalable. We employed BARAC for live cell fluorescence imaging of azide-labeled glycans. The high signal-to-background ratio obtained using nanomolar concentrations of BARAC obviated the need for washing steps. Thus, BARAC is a promising reagent for in vivo imaging.
Figures

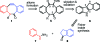

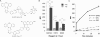

References
-
- Prescher J. A.; Bertozzi C. R. Nat. Chem. Biol. 2005, 1, 13–21. - PubMed
-
- Kele P.; Li X.; Link M.; Nagy K.; Herner A.; Lorincz K.; Beni S.; Wolfbeis O. S. Org. Biomol. Chem. 2009, 7, 3486–3490. - PubMed
-
- Wolbers F.; ter Braak P.; Le Gac S.; Luttge R.; Andersson H.; Vermes I.; van den Berg A. Electrophoresis 2006, 27, 5073. - PubMed
-
-
For a recent review of all bioorthogonal Cu-free click cycloadditions, see:
- Jewett J. C.; Bertozzi C. R.. Chem. Soc. Rev.2010, in press DOI:10.1039/B901970G.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

