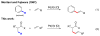Pd(II)-catalyzed olefination of sp3 C-H bonds
- PMID: 20187642
- PMCID: PMC2841429
- DOI: 10.1021/ja1010866
Pd(II)-catalyzed olefination of sp3 C-H bonds
Abstract
The first Pd(II)-catalyzed sp(3) C-H olefination reaction has been developed using N-arylamide directing groups. Following olefination, the resulting intermediates were found to undergo rapid 1,4-addition to give the corresponding gamma-lactams. Notably, this method was effective with substrates containing alpha-hydrogen atoms and could be applied to effect methylene C-H olefination of cyclopropane substrates.
Figures
References
-
-
For reviews of the Mizoroki–Heck reaction, see: Heck RF. Org React. 1982;27:345.Tsuji J. Palladium Reagents and Catalysts. Wiley; Chichester, UK: 1995. Chapter 4.Bräse S, de Meijere A. In: Metal-Catalyzed Cross-Coupling Reactions. de Meijere A, Diederich F, editors. Wiley-VCH; New York: 2004. Chapter 5.Nicolaou KC, Bulger PG, Sarlah D. Angew Chem, Int Ed. 2005;44:4442.
-
-
- Moritani I, Fujiwara Y. Tetrahedron Lett. 1967:1119.
- Jia C, Kitamura T, Fujiwara Y. Acc Chem Res. 2001;34:633. - PubMed
-
- Itahara T. Synthesis. 1979:151.
- Ferreira EM, Stoltz BM. J Am Chem Soc. 2003;125:9578. - PubMed
- Ma S, Yu S. Tetrahedron Lett. 2004;45:8419.
- Liu C, Widenhoefer RA. J Am Chem Soc. 2004;126:10250. - PubMed
- Grimster NP, Gauntlett C, Godfrey CRA, Gaunt MJ. Angew Chem, Int Ed. 2005;44:3125. - PubMed
- Capito E, Brown JM, Ricci A. Chem Commun. 2005:1854. - PubMed
-
- Miura M, Tsuda T, Satoh T, Pivsa-Art S, Nomura M. J Org Chem. 1998;63:5211.
- Boele MDK, van Strijdonck GTPF, de Vries AHM, Kamer PCJ, de Vries JG, van Leeuwen PWNM. J Am Chem Soc. 2002;124:1586. - PubMed
- Zaitsev VG, Daugulis O. J Am Chem Soc. 2005;127:4156. - PubMed
- Wang DH, Engle KM, Shi BF, Yu JQ. Science. 2010;327:315. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources