Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine
- PMID: 20187746
- PMCID: PMC2922992
- DOI: 10.1086/651147
Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine
Abstract
To date, no vaccine is available to prevent human schistosomiasis. We have targeted a protein of Schistosoma mansoni that plays an important role in the surface membrane renewal process, a mechanism widely believed to be utilized by the parasite as an immune evasion strategy. Sm-p80 antigen is a promising vaccine target because of its documented immunogenicity, protective efficacy, and antifecundity effects observed in both experimental murine and nonhuman primate models of this infectious disease. In the present study, we report that, in a vector approved for human use (VR1020), an Sm-p80-based DNA vaccine formulation confers a 46% reduction in the worm burden in a baboon (Papio anubis) model. Baboons vaccinated with Sm-p80-VR1020 had a 28% decrease in egg production after challenge with the infectious parasite. Sm-p80-VR1020 vaccine elicited robust immune responses to specific antigen Sm-p80, including immunoglobulin (Ig) G, its subtypes IgG1 and IgG2, and IgA and IgM in vaccinated animals. When stimulated in vitro with recombinant Sm-p80, peripheral blood mononuclear cells and splenocytes from baboons vaccinated with Sm-p80-VR1020 produced considerably higher levels of T helper 1 response-enhancing cytokines (interleukin [IL]-2 and interferon-gamma) than T helper 2 (Th2) response-enhancing cytokines (IL-4 and IL-10). Peripheral blood mononuclear cells produced a significantly higher number of spot-forming units for interferon-gamma than for IL-4 in enzyme-linked immunosorbent spot assays. A mixed T helper 1/T helper 2 type of humoral and T cell responses was generated after immunization with Sm-p80-VR1020. These findings again highlight the potential of Sm-p80 as a promising vaccine candidate for schistosomiasis.
Conflict of interest statement
Authors do not have any commercial or other associations that might pose a conflict of interest.
Figures
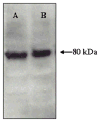


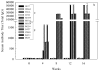




References
-
- van der Werf MJ, de Vlas SJ, Brooker S, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86(2–3):125–39. - PubMed
-
- Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357(10):1018–27. - PubMed
-
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. - PubMed
-
- Doenhoff MJ, Hagan P, Cioli D, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

