Constitutive activation of BMP signalling abrogates experimental metastasis of OVCA429 cells via reduced cell adhesion
- PMID: 20187934
- PMCID: PMC2838885
- DOI: 10.1186/1757-2215-3-5
Constitutive activation of BMP signalling abrogates experimental metastasis of OVCA429 cells via reduced cell adhesion
Abstract
Background: Activation of bone morphogenetic protein (BMP)4 signalling in human ovarian cancer cells induces a number of phenotypic changes in vitro, including altered cell morphology, adhesion, motility and invasion, relative to normal human ovarian surface epithelial cells. From these in vitro analyses, we had hypothesized that active BMP signalling promotes the metastatic potential of ovarian cancer.
Methods: To test this directly, we engineered OVCA429 human ovarian cancer cells possessing doxycycline-inducible expression of a constitutively-active mutant BMP receptor, ALK3QD, and administered these cells to immunocompromised mice. Further characterization was performed in vitro to address the role of activated BMP signalling on the EOC phenotype, with particular emphasis on epithelial-mesenchymal transition (EMT) and cell adhesion.
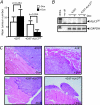
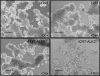
Results: Unexpectedly, doxycycline-induced ALK3QD expression in OVCA429 cells reduced tumour implantation on peritoneal surfaces and ascites formation when xenografted into immunocompromised mice by intraperitoneal injection. To determine the potential mechanisms controlling this in vivo observation, we followed with several cell culture experiments. Doxycycline-induced ALK3QD expression enhanced the refractile, spindle-shaped morphology of cultured OVCA429 cells eliciting an EMT-like response. Using in vitro wound healing assays, we observed that ALK3QD-expressing cells migrated with long, cytoplasmic projections extending into the wound space. The phenotypic alterations of ALK3QD-expressing cells correlated with changes in specific gene expression patterns of EMT, including increased Snail and Slug and reduced E-cadherin mRNA expression. In addition, ALK3QD signalling reduced beta1- and beta3-integrin expression, critical molecules involved in ovarian cancer cell adhesion. The combination of reduced E-cadherin and beta-integrin expression correlates directly with the reduced EOC cell cohesion in spheroids and reduced cell adhesion to the extracellular matrix substrates fibronectin and vitronectin that was observed.
Conclusions: We propose that the key steps of ovarian cancer metastasis, specifically cell cohesion of multicellular aggregates in ascites and cell adhesion for reattachment to secondary sites, may be inhibited by overactive BMP signalling, thereby decreasing the ultimate malignant potential of ovarian cancer in this model system.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

