Rationale and design of the randomised clinical trial comparing early medication change (EMC) strategy with treatment as usual (TAU) in patients with major depressive disorder--the EMC trial
- PMID: 20187947
- PMCID: PMC2837649
- DOI: 10.1186/1745-6215-11-21
Rationale and design of the randomised clinical trial comparing early medication change (EMC) strategy with treatment as usual (TAU) in patients with major depressive disorder--the EMC trial
Abstract
Background: In major depressive disorder (MDD), the traditional belief of a delayed onset of antidepressants' effects has lead to the concept of current guidelines that treatment durations should be between 3-8 weeks before medication change in case of insufficient outcome. Post hoc analyses of clinical trials, however, have shown that improvement usually occurs within the first 10-14 days of treatment and that such early improvement (Hamilton Depression Rating Scale [HAMD] decrease >or=20%) has a substantial predictive value for final treatment outcome. Even more important, non-improvement (HAMD decrease <20%) after 14 days of treatment was found to be highly predictive for a poor final treatment outcome.
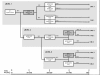
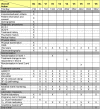
Methods/design: The EMC trial is a phase IV, multi-centre, multi-step, randomized, observer-blinded, actively controlled parallel-group clinical trial to investigate for the first time prospectively, whether non-improvers after 14 days of antidepressant treatment with an early medication change (EMC) are more likely to attain remission (HAMD-17 <or=7) on treatment day 56 compared to patients treated according to current guideline recommendation (treatment as usual; TAU). In level 1 of the EMC trial, non-improvers after 14 days of antidepressant treatment will be randomised to an EMC strategy or TAU. The EMC strategy for this study schedules a first medication change on day 15; in case of non-improvement between days 15-28, a second medication change will be performed. TAU schedules the first medication change after 28 days in case of non-response (HAMD-17 decrease <50%). Both interventions will last 42 days. In levels 2 and 3, EMC strategies will be compared with TAU strategies in improvers on day 14, who experience a stagnation of improvement during the course of treatment. The trial is supported by the German Federal Ministry of Education and Research (BMBF) and will be conducted in cooperation with the BMBF funded Interdisciplinary Centre Clinical Trials (IZKS) at the University Medical Centre Mainz and at six clinical trial sites in Germany.
Discussion: If the EMC strategies lead to significantly more remitters, changes of clinical practice, guidelines for the treatment of MDD as well as research settings can be expected.
Trial registration: Clincaltrials.gov Identifier: NCT00974155; EudraCT: 2008-008280-96.
Figures
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Washington DC: American Psychiatric Association; 1994.
-
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lépine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martínez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacín C, Romera B, Taub N, Vollebergh WA. ESEMeD/MHEDEA 2000 Investigators, European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatrica Scandinavica Supplement. 2004;420:21–27. - PubMed
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) The Journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. - DOI - PubMed
-
- Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. Journal of Mental Health and Policy and Economics. 2006;9:87–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

