Antimicrobial peptides bind more strongly to membrane pores
- PMID: 20188066
- PMCID: PMC2885479
- DOI: 10.1016/j.bbamem.2010.02.023
Antimicrobial peptides bind more strongly to membrane pores
Abstract
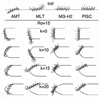
Antimicrobial peptides (AMPs) are small, usually cationic peptides, which permeabilize bacterial membranes. Understanding their mechanism of action might help design better antibiotics. Using an implicit membrane model, modified to include pores of different shapes, we show that four AMPs (alamethicin, melittin, a magainin analogue, MG-H2, and piscidin 1) bind more strongly to membrane pores, consistent with the idea that they stabilize them. The effective energy of alamethicin in cylindrical pores is similar to that in toroidal pores, whereas the effective energy of the other three peptides is lower in toroidal pores. Only alamethicin intercalates into the membrane core; MG-H2, melittin and piscidin are located exclusively at the hydrophobic/hydrophilic interface. In toroidal pores, the latter three peptides often bind at the edge of the pore, and are in an oblique orientation. The calculated binding energies of the peptides are correlated with their hemolytic activities. We hypothesize that one distinguishing feature of AMPs may be the fact that they are imperfectly amphipathic which allows them to bind more strongly to toroidal pores. An initial test on a melittin-based mutant seems to support this hypothesis.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

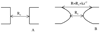





Similar articles
-
Antimicrobial peptides in toroidal and cylindrical pores.Biochim Biophys Acta. 2010 Aug;1798(8):1485-93. doi: 10.1016/j.bbamem.2010.04.004. Epub 2010 Apr 18. Biochim Biophys Acta. 2010. PMID: 20403332 Free PMC article.
-
Charge distribution and imperfect amphipathicity affect pore formation by antimicrobial peptides.Biochim Biophys Acta. 2012 May;1818(5):1274-83. doi: 10.1016/j.bbamem.2012.01.016. Epub 2012 Jan 25. Biochim Biophys Acta. 2012. PMID: 22290189 Free PMC article.
-
Free Energy Analysis of Peptide-Induced Pore Formation in Lipid Membranes by Bridging Atomistic and Coarse-Grained Simulations.J Phys Chem B. 2024 Sep 12;128(36):8737-8752. doi: 10.1021/acs.jpcb.4c03276. Epub 2024 Aug 29. J Phys Chem B. 2024. PMID: 39207202
-
The Mechanisms of Action of Cationic Antimicrobial Peptides Refined by Novel Concepts from Biophysical Investigations.Adv Exp Med Biol. 2019;1117:33-64. doi: 10.1007/978-981-13-3588-4_4. Adv Exp Med Biol. 2019. PMID: 30980352 Review.
-
Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin.J Membr Biol. 1997 Apr 1;156(3):197-211. doi: 10.1007/s002329900201. J Membr Biol. 1997. PMID: 9096062 Review. No abstract available.
Cited by
-
Antioxidant and Antimicrobial Peptides Derived from Food Proteins.Molecules. 2022 Feb 16;27(4):1343. doi: 10.3390/molecules27041343. Molecules. 2022. PMID: 35209132 Free PMC article. Review.
-
Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface.Clin Microbiol Rev. 2019 May 29;32(3):e00138-18. doi: 10.1128/CMR.00138-18. Print 2019 Jun 19. Clin Microbiol Rev. 2019. PMID: 31142499 Free PMC article. Review.
-
The effect of membrane curvature on the conformation of antimicrobial peptides: implications for binding and the mechanism of action.Eur Biophys J. 2011 Apr;40(4):545-53. doi: 10.1007/s00249-011-0677-4. Epub 2011 Jan 26. Eur Biophys J. 2011. PMID: 21267557 Free PMC article.
-
Transmission electron microscopic morphological study and flow cytometric viability assessment of Acinetobacter baumannii susceptible to Musca domestica cecropin.ScientificWorldJournal. 2014;2014:657536. doi: 10.1155/2014/657536. Epub 2014 May 5. ScientificWorldJournal. 2014. PMID: 24883421 Free PMC article.
-
3D hydrophobic moment vectors as a tool to characterize the surface polarity of amphiphilic peptides.Biophys J. 2014 Jun 3;106(11):2385-94. doi: 10.1016/j.bpj.2014.04.020. Biophys J. 2014. PMID: 24896117 Free PMC article.
References
-
- Hancock REW. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001;1:156–164. - PubMed
-
- Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006;18:24–30. - PubMed
-
- Brogden K. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. - PubMed
-
- Bechinger B. Structure and function of membrane-lytic peptides. Crit. Rev. Plant Sci. 2004;23:271–292.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

