The role of PI3P phosphatases in the regulation of autophagy
- PMID: 20188094
- PMCID: PMC2885894
- DOI: 10.1016/j.febslet.2010.02.054
The role of PI3P phosphatases in the regulation of autophagy
Abstract
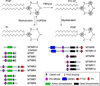
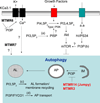
Autophagy initiation is strictly dependent on phosphatidylinositol 3-phosphate (PI3P) synthesis. PI3P production is under tight control of PI3Kinase, hVps34, in complex with Beclin-1. Mammalian cells express several PI3P phosphatases that belong to the myotubularin family. Even though some of them have been linked to serious human diseases, their cellular function is largely unknown. Two recent studies indicate that PI3P metabolism involved in autophagy initiation is further regulated by the PI3P phosphatases Jumpy and MTMR3. Additional pools of PI3P, upstream of mTOR and on the endocytic pathway, may modulate autophagy indirectly, suggesting that other PI3P phosphatases might be involved in this process. This review sums up our knowledge on PI3P phosphatases and discusses the recent progress on their role in autophagy.
Published by Elsevier B.V.
Figures
Similar articles
-
Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis.PLoS One. 2013 Oct 3;8(10):e76405. doi: 10.1371/journal.pone.0076405. eCollection 2013. PLoS One. 2013. PMID: 24098492 Free PMC article.
-
Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics.FEBS J. 2017 May;284(9):1267-1278. doi: 10.1111/febs.13987. Epub 2017 Jan 5. FEBS J. 2017. PMID: 27973739 Review.
-
PI3P phosphatase activity is required for autophagosome maturation and autolysosome formation.EMBO Rep. 2014 Sep;15(9):973-81. doi: 10.15252/embr.201438618. Epub 2014 Aug 14. EMBO Rep. 2014. PMID: 25124690 Free PMC article.
-
Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway.Hum Mol Genet. 2000 Sep 22;9(15):2223-9. doi: 10.1093/oxfordjournals.hmg.a018913. Hum Mol Genet. 2000. PMID: 11001925
-
Regulation of membrane biogenesis in autophagy via PI3P dynamics.Semin Cell Dev Biol. 2010 Sep;21(7):671-6. doi: 10.1016/j.semcdb.2010.04.002. Epub 2010 Apr 18. Semin Cell Dev Biol. 2010. PMID: 20403452 Review.
Cited by
-
Autophagy in Chronic Kidney Diseases.Kidney Dis (Basel). 2016 Apr;2(1):37-45. doi: 10.1159/000444841. Epub 2016 Mar 24. Kidney Dis (Basel). 2016. PMID: 27536690 Free PMC article. Review.
-
Uropathogenic Escherichia coli Subverts Host Autophagic Defenses by Stalling Preautophagosomal Structures to Escape Lysosome Exocytosis.J Infect Dis. 2024 Sep 23;230(3):e548-e558. doi: 10.1093/infdis/jiae063. J Infect Dis. 2024. PMID: 38330453 Free PMC article.
-
Class I PI3K Provide Lipid Substrate in T Cell Autophagy Through Linked Activity of Inositol Phosphatases.Front Cell Dev Biol. 2021 Aug 12;9:709398. doi: 10.3389/fcell.2021.709398. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458267 Free PMC article.
-
Beyond autophagy: LC3-associated phagocytosis and endocytosis.Sci Adv. 2022 Oct 28;8(43):eabn1702. doi: 10.1126/sciadv.abn1702. Epub 2022 Oct 26. Sci Adv. 2022. PMID: 36288309 Free PMC article. Review.
-
Selective autophagy: talking with the UPS.Cell Biochem Biophys. 2013 Sep;67(1):3-13. doi: 10.1007/s12013-013-9623-7. Cell Biochem Biophys. 2013. PMID: 23709310 Free PMC article. Review.
References
-
- Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3'-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. - PubMed
-
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

