The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution
- PMID: 20188109
- PMCID: PMC2866118
- DOI: 10.1016/j.jmb.2010.02.036
The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution
Abstract
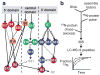
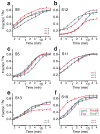
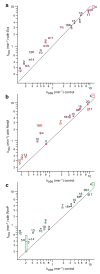
Ribosome biogenesis is facilitated by a growing list of assembly cofactors, including helicases, GTPases, chaperones, and other proteins, but the specific functions of many of these assembly cofactors are still unclear. The effect of three assembly cofactors on 30S ribosome assembly was determined in vitro using a previously developed mass-spectrometry-based method that monitors the rRNA binding kinetics of ribosomal proteins. The essential GTPase Era caused several late-binding proteins to bind rRNA faster when included in a 30S reconstitution. RimP enabled faster binding of S9 and S19 and inhibited the binding of S12 and S13, perhaps by blocking those proteins' binding sites. RimM caused proteins S5 and S12 to bind dramatically faster. These quantitative kinetic data provide important clues about the roles of these assembly cofactors in the mechanism of 30S biogenesis.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Assembly of bacterial ribosomes.Annu Rev Biochem. 2011;80:501-26. doi: 10.1146/annurev-biochem-062608-160432. Annu Rev Biochem. 2011. PMID: 21529161 Review.
-
Kinetic cooperativity in Escherichia coli 30S ribosomal subunit reconstitution reveals additional complexity in the assembly landscape.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5417-22. doi: 10.1073/pnas.0912007107. Epub 2010 Mar 5. Proc Natl Acad Sci U S A. 2010. PMID: 20207951 Free PMC article.
-
The PRC-barrel domain of the ribosome maturation protein RimM mediates binding to ribosomal protein S19 in the 30S ribosomal subunits.RNA. 2004 Nov;10(11):1798-812. doi: 10.1261/rna.7720204. RNA. 2004. PMID: 15496525 Free PMC article.
-
Escherichia coli rimM and yjeQ null strains accumulate immature 30S subunits of similar structure and protein complement.RNA. 2013 Jun;19(6):789-802. doi: 10.1261/rna.037523.112. Epub 2013 Apr 23. RNA. 2013. PMID: 23611982 Free PMC article.
-
[Ribosome: lessons of a molecular factory construction].Mol Biol (Mosk). 2014 Jul-Aug;48(4):543-60. Mol Biol (Mosk). 2014. PMID: 25842841 Review. Russian.
Cited by
-
Mutations of ribosomal protein S5 suppress a defect in late-30S ribosomal subunit biogenesis caused by lack of the RbfA biogenesis factor.RNA. 2015 Aug;21(8):1454-68. doi: 10.1261/rna.051383.115. Epub 2015 Jun 18. RNA. 2015. PMID: 26089326 Free PMC article.
-
Structural basis for the function of a small GTPase RsgA on the 30S ribosomal subunit maturation revealed by cryoelectron microscopy.Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13100-5. doi: 10.1073/pnas.1104645108. Epub 2011 Jul 25. Proc Natl Acad Sci U S A. 2011. PMID: 21788480 Free PMC article.
-
On an algorithmic definition for the components of the minimal cell.PLoS One. 2018 Jun 1;13(6):e0198222. doi: 10.1371/journal.pone.0198222. eCollection 2018. PLoS One. 2018. PMID: 29856803 Free PMC article.
-
Overproduction of a Dominant Mutant of the Conserved Era GTPase Inhibits Cell Division in Escherichia coli.J Bacteriol. 2020 Oct 8;202(21):e00342-20. doi: 10.1128/JB.00342-20. Print 2020 Oct 8. J Bacteriol. 2020. PMID: 32817092 Free PMC article.
-
A combined quantitative mass spectrometry and electron microscopy analysis of ribosomal 30S subunit assembly in E. coli.Elife. 2014 Oct 14;3:e04491. doi: 10.7554/eLife.04491. Elife. 2014. PMID: 25313868 Free PMC article.
References
-
- Bremer H, Dennis PP. Modulation of Chemical Composition and Other Parameters of the Cell by Growth Rate. In: Neidhardt FC, editor. Escherichia Coli and Salmonella: Cellular and Molecular Biology. 2nd. American Society for Microbiology; Washington, DC: 1996. pp. 1553–1569.
-
- Dez C, Tollervey D. Ribosome synthesis meets the cell cycle. Curr Opin Microbiol. 2004;7:631–7. - PubMed
-
- Wilson DN, Nierhaus KH. The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol. 2007;42:187–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

