Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons
- PMID: 20188150
- PMCID: PMC3315124
- DOI: 10.1016/j.neuroscience.2010.02.037
Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons
Abstract
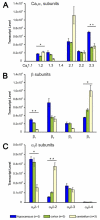
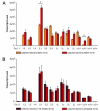
The importance and diversity of calcium signaling in the brain is mirrored by the expression of a multitude of voltage-activated calcium channel (Ca(V)) isoforms. Whereas the overall distributions of alpha(1) subunits are well established, the expression patterns of distinct channel isoforms in specific brain regions and neurons, as well as those of the auxiliary beta and alpha(2)delta subunits are still incompletely characterized. Further it is unknown whether neuronal differentiation and activity induce changes of Ca(V) subunit composition. Here we combined absolute and relative quantitative TaqMan reverse transcription PCR (RT-PCR) to analyze mRNA expression of all high voltage-activated Ca(V) alpha(1) subunits and all beta and alpha(2)delta subunits. This allowed for the first time the direct comparison of complete Ca(V) expression profiles of mouse cortex, hippocampus, cerebellum, and cultured hippocampal neurons. All brain regions expressed characteristic profiles of the full set of isoforms, except Ca(V)1.1 and Ca(V)1.4. In cortex development was accompanied by a general down regulation of alpha(1) and alpha(2)delta subunits and a shift from beta(1)/beta(3) to beta(2)/beta(4). The most abundant Ca(V) isoforms in cerebellum were Ca(V)2.1, beta(4), and alpha(2)delta-2, and in hippocampus Ca(V)2.3, beta(2), and alpha(2)delta-1. Interestingly, cultured hippocampal neurons also expressed the same Ca(V) complement as adult hippocampus. During differentiation specific Ca(V) isoforms experienced up- or down-regulation; however blocking electrical activity did not affect Ca(V) expression patterns. Correlation analysis of alpha(1), beta and alpha(2)delta subunit expression throughout all examined preparations revealed a strong preference of Ca(V)2.1 for beta(4) and alpha(2)delta-2 and vice versa, whereas the other alpha(1) isoforms were non-selectively expressed together with each of the other beta and alpha(2)delta isoforms. Together our results revealed a remarkably stable overall Ca(2+) channel complement as well as tissue specific differences in expression levels. Developmental changes are likely determined by an intrinsic program and not regulated by changes in neuronal activity.
Copyright 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol. 2003;13:298–307. - PubMed
-
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002;43:1595–1603. - PubMed
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. - PMC - PubMed
-
- Barnes S, Kelly ME. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol. 2002;514:465–476. - PubMed
-
- Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

