HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide
- PMID: 20188164
- PMCID: PMC2873898
- DOI: 10.1016/j.freeradbiomed.2010.02.023
HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide
Abstract
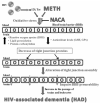
An increased risk of HIV-1 associated dementia (HAD) has been observed in patients abusing methamphetamine (METH). Since both HIV viral proteins (gp120, Tat) and METH induce oxidative stress, drug abusing patients are at a greater risk of oxidative stress-induced damage. The objective of this study was to determine if N-acetylcysteine amide (NACA) protects the blood brain barrier (BBB) from oxidative stress-induced damage in animals exposed to gp120, Tat and METH. To study this, CD-1 mice pre-treated with NACA/saline, received injections of gp120, Tat, gp120+Tat or saline for 5days, followed by three injections of METH/saline on the fifth day, and sacrificed 24h after the final injection. Various oxidative stress parameters were measured, and animals treated with gp120+Tat+Meth were found to be the most challenged group, as indicated by their GSH and MDA levels. Treatment with NACA significantly rescued the animals from oxidative stress. Further, NACA-treated animals had significantly higher expression of TJ proteins and BBB permeability as compared to the group treated with gp120+Tat+METH alone, indicating that NACA can protect the BBB from oxidative stress-induced damage in gp120, Tat and METH exposed animals, and thus could be a viable therapeutic option for patients with HAD.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures










References
-
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11:S35. - PubMed
-
- Krebs FC, Ross H, McAllister J, Wigdahl B. HIV-1-associated central nervous system dysfunction. Adv.Pharmacol. 2000;49:315. - PubMed
-
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 2002;186:S193. - PubMed
-
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin. Lab. Med. 2002;22:703. - PubMed
-
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;7:411–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

