Systemic immunization with CCL27/CTACK modulates immune responses at mucosal sites in mice and macaques
- PMID: 20188250
- PMCID: PMC4396814
- DOI: 10.1016/j.vaccine.2009.10.095
Systemic immunization with CCL27/CTACK modulates immune responses at mucosal sites in mice and macaques
Abstract
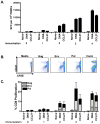
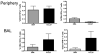
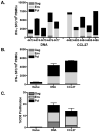
Plasmid DNA is a promising vaccine platform that has been shown to be safe and able to be administered repeatedly without vector interference. Enhancing the potency of DNA vaccination through co-delivery of molecular adjuvants is one strategy currently under investigation. Here we describe the use of the novel chemokine adjuvant CCL27/CTACK to enhance immune responses to an HIV-1 or SIV antigen in mice and rhesus macaques. CCL27 has been shown to play a role in inflammatory responses through chemotaxis of CCR10+ cells, and we hypothesized that CCL27 may modulate adaptive immune responses. Immunizations in mice with HIV-1gag/CCL27 enhanced immune responses both at peripheral and, surprisingly, at mucosal sites. To confirm these findings in a large-animal model, we created optimized CCL27 and SIV antigenic plasmid constructs for rhesus macaques. 10 macaques (n=5/group) were immunized intramuscularly with 1mg/construct of antigenic plasmids+/-CCL27 with electroporation. We observed significant IFN-gamma secretion and CD8+ T-cell proliferation in peripheral blood. Interestingly, CCL27 co-immunized macaques exhibited a trend toward greater effector CD4+ T cells in the bronchiolar lavage (BAL). CCL27 co-delivery also elicited greater antigen-specific IgA at unique sites including BAL and fecal samples but not in the periphery. Future studies incorporating CCL27 as an adjuvant in vaccine or therapy models where eliciting immune responses in the lung are warranted.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures


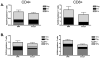

Similar articles
-
Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit.Vaccine. 2010 Feb 23;28(8):1962-74. doi: 10.1016/j.vaccine.2009.10.099. Vaccine. 2010. PMID: 20188252 Free PMC article.
-
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge.J Virol. 2014 Sep 1;88(17):9579-89. doi: 10.1128/JVI.00975-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920805 Free PMC article.
-
Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.J Virol. 2017 Jan 31;91(4):e01844-16. doi: 10.1128/JVI.01844-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27928002 Free PMC article.
-
Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model.Vaccine. 2006 Mar 10;24(11):1811-20. doi: 10.1016/j.vaccine.2005.10.026. Epub 2005 Oct 25. Vaccine. 2006. PMID: 16274888
-
The role of immunity in protection from mucosal SIV infection in macaques.Oral Dis. 2002;8 Suppl 2:63-8. doi: 10.1034/j.1601-0825.2002.00014.x. Oral Dis. 2002. PMID: 12164663 Review.
Cited by
-
Cytokines Elevated in HIV Elite Controllers Reduce HIV Replication In Vitro and Modulate HIV Restriction Factor Expression.J Virol. 2017 Feb 28;91(6):e02051-16. doi: 10.1128/JVI.02051-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053103 Free PMC article.
-
DNA-based immunotherapy for cancer: In vivo approaches for recalcitrant targets.Mol Ther. 2025 Jun 4;33(6):2719-2739. doi: 10.1016/j.ymthe.2025.04.008. Epub 2025 Apr 9. Mol Ther. 2025. PMID: 40211538 Review.
-
Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes.Plant Biotechnol J. 2016 Nov;14(11):2190-2200. doi: 10.1111/pbi.12575. Epub 2016 Jun 1. Plant Biotechnol J. 2016. PMID: 27155248 Free PMC article.
-
CCL27: Novel Cytokine with Potential Role in Pathogenesis of Multiple Sclerosis.Biomed Res Int. 2015;2015:189638. doi: 10.1155/2015/189638. Epub 2015 Jul 29. Biomed Res Int. 2015. PMID: 26295034 Free PMC article.
-
Generation of antigen-specific immunity following systemic immunization with DNA vaccine encoding CCL25 chemokine immunoadjuvant.Hum Vaccin Immunother. 2012 Nov 1;8(11):1607-19. doi: 10.4161/hv.22574. Epub 2012 Nov 1. Hum Vaccin Immunother. 2012. PMID: 23151454 Free PMC article.
References
-
- Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer seminars in immunopathology. 2006 Nov;28(3):267–79. - PubMed
-
- Gao F, Li Y, Decker JM, Peyerl FW, Bibollet-Ruche F, Rodenburg CM, et al. Codon usage optimization of HIV type 1 subtype C gag, pol, env, and nef genes: in vitro expression and immune responses in DNA-vaccinated mice. AIDS research and human retroviruses. 2003 Sep;19(9):817–23. - PubMed
-
- Leifert JA, Rodriguez-Carreno MP, Rodriguez F, Whitton JL. Targeting plasmid-encoded proteins to the antigen presentation pathways. Immunological reviews. 2004 Jun;199:40–53. - PubMed
-
- Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods (San Diego, Calif. 2006 Sep;40(1):86–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

