Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit
- PMID: 20188252
- PMCID: PMC2830913
- DOI: 10.1016/j.vaccine.2009.10.099
Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit
Abstract
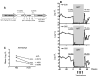
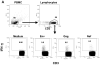
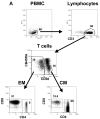
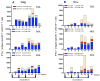
We have previously reported that therapeutic immunization by intramuscular injection of optimized plasmid DNAs encoding SIV antigens effectively induces immune responses able to reduce viremia in antiretroviral therapy (ART)-treated SIVmac251-infected Indian rhesus macaques. We subjected such therapeutically immunized macaques to a second round of therapeutic vaccination using a combination of plasmids expressing SIV genes and the IL-15/IL-15 receptor alpha as molecular adjuvant, which were delivered by the more efficacious in vivo constant-current electroporation. A very strong induction of antigen-specific responses to Gag, Env, Nef, and Pol, during ART (1.2-1.6% of SIV-specific T cells in the circulating T lymphocytes) was obtained with the improved vaccination method. Immunological responses were characterized by the production of IFN-gamma, IL-2, and TNF-alpha either alone, or in combination as double or triple cytokine positive multifunctional T cells. A significant induction of CD4(+) T cell responses, mainly targeting Gag, Nef, and Pol, as well as of CD8(+) T cells, mainly targeting Env, was found in both T cells with central memory and effector memory markers. After release from ART, the animals showed a virological benefit with a further approximately 1 log reduction in viremia. Vaccination with plasmid DNAs has several advantages over other vaccine modalities, including the possibility for repeated administration, and was shown to induce potent, efficacious, and long-lasting recall immune responses. Therefore, these data support the concept of adding DNA vaccination to the HAART regimen to boost the HIV-specific immune responses.
Published by Elsevier Ltd.
Figures




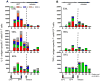

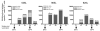
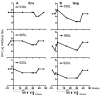
References
-
- Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. - PubMed
-
- Andrieu JM, Lu W. A dendritic cell-based vaccine for treating HIV infection: background and preliminary results. J Intern Med. 2007;261:123–131. - PubMed
-
- Bansal A, Jackson B, West K, Wang S, Lu S, Kennedy JS, Goepfert PA. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol. 2008;82:6458–6469. - PMC - PubMed
-
- Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN. Intracellular interaction of interleukin IL-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2007;283:4189–4199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

