The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision
- PMID: 20188572
- PMCID: PMC2891588
- DOI: 10.1016/j.tibs.2010.01.005
The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision
Abstract
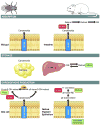
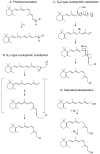
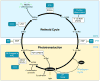
Recently, much progress has been made in elucidating the chemistry and metabolism of retinoids and carotenoids, as well as the structures of processing proteins related to vision. Carotenoids and their retinoid metabolites are isoprenoids, so only a limited number of chemical transformations are possible, and just a few of these occur naturally. Although there is an intriguing evolutionary conservation of the key components involved in the production and recycling of chromophores, these genes have also adapted to the specific requirements of insect and vertebrate vision. These 'ancestral footprints' in animal genomes bear witness to the common origin of the chemistry of vision, and will further stimulate research across evolutionary boundaries.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
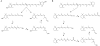


References
-
- Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. - PubMed
-
- Bitensky MW, et al. The mechanism of activation of light-activated phosphodiesterase and evidence for homology with hormone-activated adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:227–237. - PubMed
-
- McBee JK, et al. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Progress in retinal and eye research. 2001;20:469–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

