Modulation of visual responses by behavioral state in mouse visual cortex
- PMID: 20188652
- PMCID: PMC3184003
- DOI: 10.1016/j.neuron.2010.01.033
Modulation of visual responses by behavioral state in mouse visual cortex
Abstract
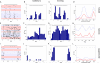
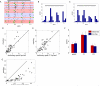
Studies of visual processing in rodents have conventionally been performed on anesthetized animals, precluding examination of the effects of behavior on visually evoked responses. We have now studied the response properties of neurons in primary visual cortex of awake mice that were allowed to run on a freely rotating spherical treadmill with their heads fixed. Most neurons showed more than a doubling of visually evoked firing rate as the animal transitioned from standing still to running, without changes in spontaneous firing or stimulus selectivity. Tuning properties in the awake animal were similar to those measured previously in anesthetized animals. Response magnitude in the lateral geniculate nucleus did not increase with locomotion, demonstrating that the striking change in responsiveness did not result from peripheral effects at the eye. Interestingly, some narrow-spiking cells were spontaneously active during running but suppressed by visual stimuli. These results demonstrate powerful cell-type-specific modulation of visual processing by behavioral state in awake mice.
Figures


References
-
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. - PubMed
-
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. - PubMed
-
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

