A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier
- PMID: 20188654
- PMCID: PMC2831809
- DOI: 10.1016/j.neuron.2010.02.004
A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier
Abstract
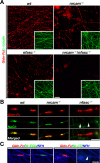
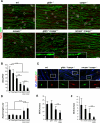
Saltatory conduction requires high-density accumulation of Na(+) channels at the nodes of Ranvier. Nodal Na(+) channel clustering in the peripheral nervous system is regulated by myelinating Schwann cells through unknown mechanisms. During development, Na(+) channels are first clustered at heminodes that border each myelin segment, and later in the mature nodes that are formed by the fusion of two heminodes. Here, we show that initial clustering of Na(+) channels at heminodes requires glial NrCAM and gliomedin, as well as their axonal receptor neurofascin 186 (NF186). We further demonstrate that heminodal clustering coincides with a second, paranodal junction (PNJ)-dependent mechanism that allows Na(+) channels to accumulate at mature nodes by restricting their distribution between two growing myelin internodes. We propose that Schwann cells assemble the nodes of Ranvier by capturing Na(+) channels at heminodes and by constraining their distribution to the nodal gap. Together, these two cooperating mechanisms ensure fast and efficient conduction in myelinated nerves.
Figures






References
-
- Arroyo EJ, Sirkowski EE, Chitale R, Scherer SS. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479:424–434. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr., Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
-
- Berthold CH, Rydmark M. Electron microscopic serial section analysis of nodes of Ranvier in lumbosacral spinal roots of the cat: ultrastructural organization of nodal compartments in fibres of different sizes. J Neurocytol. 1983;12:475–505. - PubMed
-
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. - PubMed
-
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

