A role for Gcn5 in replication-coupled nucleosome assembly
- PMID: 20188666
- PMCID: PMC2954627
- DOI: 10.1016/j.molcel.2010.01.020
A role for Gcn5 in replication-coupled nucleosome assembly
Abstract
Acetylation of lysine residues at the H3 N terminus is proposed to function in replication-coupled (RC) nucleosome assembly, a process critical for the inheritance of epigenetic information and maintenance of genome stability. However, the role of H3 N-terminal lysine acetylation and the corresponding lysine acetyltransferase (KAT) in RC nucleosome assembly are not known. Here we show that Gcn5, a KAT that functions in transcription, works in parallel with Rtt109, the H3 lysine 56 KAT, to promote RC nucleosome assembly. Cells lacking both Gcn5 and Rtt109 are highly sensitive to DNA damaging agents. Moreover, cells lacking GCN5 or those that express N-terminal H3 mutants are compromised for deposition of new H3 onto replicating DNA and also show reduced binding of H3 to CAF-1, a histone chaperone involved in RC nucleosome assembly. These results demonstrate that Gcn5 regulates RC nucleosome assembly, in part, by promoting H3 association with CAF-1 via H3 acetylation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

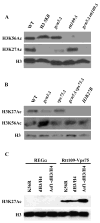
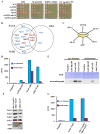


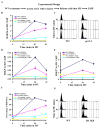
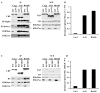
Comment in
-
The tango of histone marks and chaperones at replication fork.Mol Cell. 2010 Mar 12;37(5):595-6. doi: 10.1016/j.molcel.2010.02.019. Mol Cell. 2010. PMID: 20227364
References
-
- Ai X, Parthun MR. The nuclear Hat1p/Hat2p complex: a molecular link between type B histone acetyltransferases and chromatin assembly. Mol Cell. 2004;14:195–205. - PubMed
-
- Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J Biol Chem. 2002;277:7989–7995. - PubMed
-
- Benson LJ, Gu Y, Yakovleva T, Tong K, Barrows C, Strack CL, Cook RG, Mizzen CA, Annunziato AT. Modifications of H3 and H4 during chromatin replication, nucleosome assembly, and histone exchange. J Biol Chem. 2006;281:9287–9296. - PubMed
-
- Candau R, Berger SL. Structural and functional analysis of yeast putative adaptors. Evidence for an adaptor complex in vivo. J Biol Chem. 1996;271:5237–5245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

