Protein kinase Cgamma in colon cancer cells: expression, Thr514 phosphorylation and sensitivity to butyrate-mediated upregulation as related to the degree of differentiation
- PMID: 20188713
- PMCID: PMC2999905
- DOI: 10.1016/j.cbi.2010.02.035
Protein kinase Cgamma in colon cancer cells: expression, Thr514 phosphorylation and sensitivity to butyrate-mediated upregulation as related to the degree of differentiation
Abstract
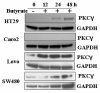
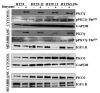
Protein kinase C (PKC) isoenzymes are expressed and activated in a cell type-specific manner, and play an essential role in tissue-specific signal transduction. The presence of butyrate at millimolar concentrations in the colon raises the question of whether it affects the expression of PKC isoenzymes in the different cell types of the colonic epithelium. We investigated the protein expression levels of PKCgamma, Thr(514)-phosphorylated PKCgamma (pPKCgamma-Thr(514)), and their subcellular distribution as affected by butyrate in a set of colon cancer cell lines. Thr(514)-phosphorylation of de novo synthesized PKCgamma is the first step in priming of the inactive PKCgamma before its release into the cytoplasm. For immunoblot analysis, we employed three antibodies, one against an unmodified sequence, mapping within 50 amino acids at its C-terminus, a second against pPKCgamma-Thr(514), and a third against pPKCgamma-pan-Thr(514). The antibody against an unmodified C-terminal peptide epitope did not recognize pPKCgamma-Thr(514), suggesting that phosphorylation at this site interferes with the binding of the antibody to the C-terminus. Marked butyrate-induced upregulation of PKCgamma occurred in HT29 cells (model for colonocyte stem cells) and HT29-derived cell lines. However, in Caco2 and IEC-18 cells (models for differentiated intestinal epithelial cells), PKCgamma was insensitive to upregulation, and present exclusively as pPKCgamma-Thr(514). Lovo and SW480 expressed higher levels of PKCgamma. In HT29 cells, butyrate-induced upregulation of the non-phosphorylated PKCgamma was observed in both the membrane and the cytosolic fraction. In Caco2 cells, the Thr(514)-phosphorylated form was present at high levels in both fractions. The presence of unphosphorylated PKCgamma in HT29 cells, and its complete absence in Caco2 cells demonstrates a cell type-dependent differential coupling of Thr(514)-phosphorylation with de novo synthesis of PKCgamma in colon cancer cells.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures





Similar articles
-
Butyrate-induced alterations of phosphoinositide metabolism, protein kinase C activity and reduced CD44 variant expression in HT-29 colon cancer cells.Int J Mol Med. 2009 May;23(5):639-49. doi: 10.3892/ijmm_00000175. Int J Mol Med. 2009. PMID: 19360323
-
LysoPCs induce Hck- and PKCδ-mediated activation of PKCγ causing p47phox phosphorylation and membrane translocation in neutrophils.J Leukoc Biol. 2017 Jan;101(1):261-273. doi: 10.1189/jlb.3A0813-420RRR. Epub 2016 Aug 16. J Leukoc Biol. 2017. PMID: 27531930 Free PMC article.
-
Post-translational modulation of CD133 expression during sodium butyrate-induced differentiation of HT29 human colon cancer cells: implications for its detection.J Cell Physiol. 2010 Jul;224(1):234-41. doi: 10.1002/jcp.22124. J Cell Physiol. 2010. PMID: 20333645
-
Butyrate-induced differentiation of colon cancer cells is PKC and JNK dependent.Dig Dis Sci. 2005 Mar;50(3):490-8. doi: 10.1007/s10620-005-2463-6. Dig Dis Sci. 2005. PMID: 15810631
-
Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre.Mutat Res. 2009 Jul-Aug;682(1):39-53. doi: 10.1016/j.mrrev.2009.04.001. Epub 2009 Apr 19. Mutat Res. 2009. PMID: 19383551 Review.
Cited by
-
Olive Pâté by Multi-Phase Decanter as Potential Source of Bioactive Compounds of Both Nutraceutical and Anticancer Effects.Molecules. 2020 Dec 16;25(24):5967. doi: 10.3390/molecules25245967. Molecules. 2020. PMID: 33339392 Free PMC article.
-
Protein Kinase C at the Crossroad of Mutations, Cancer, Targeted Therapy and Immune Response.Biology (Basel). 2023 Jul 26;12(8):1047. doi: 10.3390/biology12081047. Biology (Basel). 2023. PMID: 37626933 Free PMC article. Review.
-
Thienoquinolines as novel disruptors of the PKCε/RACK2 protein-protein interaction.J Med Chem. 2014 Apr 24;57(8):3235-46. doi: 10.1021/jm401605c. Epub 2014 Apr 8. J Med Chem. 2014. PMID: 24712764 Free PMC article.
-
A Proteomics Analysis Reveals 9 Up-Regulated Proteins Associated with Altered Cell Signaling in Colon Cancer Patients.Protein J. 2017 Dec;36(6):513-522. doi: 10.1007/s10930-017-9746-6. Protein J. 2017. PMID: 29128960
-
DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia.Strahlenther Onkol. 2014 Sep;190(9):815-22. doi: 10.1007/s00066-014-0617-1. Epub 2014 Feb 22. Strahlenther Onkol. 2014. PMID: 24562547
References
-
- Kruh J, Defer L, Tichonicky L. Effects of butyrate on cell proliferation and gene expression. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge: 1995. pp. 275–288.
-
- Csordas A. Toxicology of butyrate and short-chain fatty acids. In: Hill MJ, editor. Role of Gut Bacteria in Human Toxicology and Pharmacology. Taylor and Francis; London: 1995. pp. 105–127.
-
- Lindemann RK, Gabrielli B, Johnstone RW. Histone-deacetylase inhibitors for the treatment of cancer. Cell Cycle. 2004;3:1779–1788. - PubMed
-
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanism of action. Oncogene. 2007;26:5541–5552. - PubMed
-
- Black JD. Protein kinase C-mediated regulation of the cell cycle. Front. Biosci. 2000;5:D406–D423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

