EPCs and pathological angiogenesis: when good cells go bad
- PMID: 20188747
- PMCID: PMC3650470
- DOI: 10.1016/j.mvr.2010.02.011
EPCs and pathological angiogenesis: when good cells go bad
Abstract
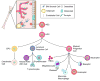
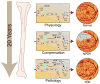
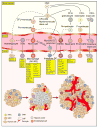
Bone-marrow-derived endothelial progenitor cells (EPCs) contribute to angiogenesis-mediated pathological neovascularization, and recent studies have begun to recognize the biological significance of this contribution. This review will discuss the ability of EPCs to contribute to neovascularization in both physiological and pathological conditions. Circulating EPCs were originally identified in 1997 by Asahara as CD34(+) VEGFR2(+) mononuclear cells. These cells differentiated into an endothelial phenotype, expressed endothelial markers, and incorporated into neovessels at sites of ischemia (Asahara et al., 1997). EPCs provide both instructive (release of pro-angiogenic cytokines) and structural (vessel incorporation and stabilization) functions that contribute to the initiation of neo-angiogenesis. EPC populations can be characterized based on surface markers of freshly isolated cells, or they can be described by their in vitro characteristics once placed in culture. However, a major stumbling block to progress in the field has been the lack of consensus among investigators as to the optimal characterization of EPCs. This review intends to address the role of both EPC classes and evaluate how they interact in the setting of pathological angiogenesis. Since the EPCs may be responsible for turning on the "angiogenic switch," strategies have been employed to keep this switch in the "off" position for diseases like cancer, retinopathy, and wet AMD. The expectation is that EPCs will evolve into clinically useful prognostic and predictive tools in cancer and in ocular diseases associated with pathological neovascularization and that targeting this cell type is a key to successful management of patients suffering from diseases associated with pathological neovascularization.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Decursin inhibits vasculogenesis in early tumor progression by suppression of endothelial progenitor cell differentiation and function.J Cell Biochem. 2012 May;113(5):1478-87. doi: 10.1002/jcb.24085. J Cell Biochem. 2012. PMID: 22298358
-
Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy.Am J Pathol. 2010 Jan;176(1):504-15. doi: 10.2353/ajpath.2010.081152. Epub 2009 Nov 30. Am J Pathol. 2010. PMID: 19948824 Free PMC article.
-
Tumor angiogenesis promoted by ex vivo differentiated endothelial progenitor cells is effectively inhibited by an angiogenesis inhibitor, TK1-2.Cancer Res. 2007 May 15;67(10):4851-9. doi: 10.1158/0008-5472.CAN-06-2979. Cancer Res. 2007. PMID: 17510415
-
Endothelial progenitor cells as a new agent contributing to vascular repair.Arch Immunol Ther Exp (Warsz). 2007 Jul-Aug;55(4):247-59. doi: 10.1007/s00005-007-0027-5. Epub 2007 Jul 23. Arch Immunol Ther Exp (Warsz). 2007. PMID: 17659378 Review.
-
The involvement of endothelial progenitor cells in tumor angiogenesis.J Cell Mol Med. 2004 Jul-Sep;8(3):294-300. doi: 10.1111/j.1582-4934.2004.tb00319.x. J Cell Mol Med. 2004. PMID: 15491505 Free PMC article. Review.
Cited by
-
Differential uPAR recruitment in caveolar-lipid rafts by GM1 and GM3 gangliosides regulates endothelial progenitor cells angiogenesis.J Cell Mol Med. 2015 Jan;19(1):113-23. doi: 10.1111/jcmm.12410. Epub 2014 Oct 14. J Cell Mol Med. 2015. PMID: 25313007 Free PMC article.
-
Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces.Clin Oral Investig. 2013 Jan;17(1):301-9. doi: 10.1007/s00784-012-0691-7. Epub 2012 Mar 10. Clin Oral Investig. 2013. PMID: 22406922
-
A module of human peripheral blood mononuclear cell transcriptional network containing primitive and differentiation markers is related to specific cardiovascular health variables.PLoS One. 2014 Apr 23;9(4):e95124. doi: 10.1371/journal.pone.0095124. eCollection 2014. PLoS One. 2014. PMID: 24759906 Free PMC article.
-
Bone marrow-derived cells in ocular neovascularization: contribution and mechanisms.Angiogenesis. 2016 Apr;19(2):107-18. doi: 10.1007/s10456-016-9497-6. Epub 2016 Feb 15. Angiogenesis. 2016. PMID: 26880135 Free PMC article. Review.
-
Clinical significance of 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester-labeled microspheres for detecting endothelial progenitor cells in human peripheral blood.Exp Ther Med. 2017 Aug;14(2):1659-1664. doi: 10.3892/etm.2017.4657. Epub 2017 Jun 23. Exp Ther Med. 2017. PMID: 28810633 Free PMC article.
References
-
- Anghelina M, et al. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665–76. - PubMed
-
- Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Barber CL, Iruela-Arispe ML. The ever-elusive endothelial progenitor cell: identities, functions and clinical implications. Pediatr Res. 2006;59:26R–32R. - PubMed
-
- Bertolini F, et al. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

