A novel platform for in situ investigation of cells and tissues under mechanical strain
- PMID: 20188869
- PMCID: PMC2883029
- DOI: 10.1016/j.actbio.2010.02.035
A novel platform for in situ investigation of cells and tissues under mechanical strain
Abstract
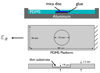
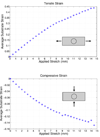
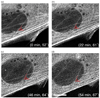
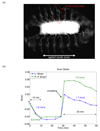
The mechanical micro-environment influences cellular responses such as migration, proliferation, differentiation and apoptosis. Cells are subjected to mechanical stretching in vivo, e.g., epithelial cells during embryogenesis. Current methodologies do not allow high-resolution in situ observation of cells and tissues under applied strain, which may reveal intracellular dynamics and the origin of cell mechanosensitivity. A novel polydimethylsiloxane substrate was developed, capable of applying tensile and compressive strain (up to 45%) to cells and tissues while allowing in situ observation with high-resolution optics. The strain field of the substrate was characterized experimentally using digital image correlation, and the deformation was modeled by the finite element method, using a Mooney-Rivlin hyperelastic constitutive relation. The substrate strain was found to be uniform for >95% of the substrate area. As a demonstration of the system, mechanical strain was applied to single fibroblasts transfected with GFP-actin and whole transgenic Drosophila embryos expressing GFP in all neurons during live imaging. Three observations of biological responses due to applied strain are reported: (1) dynamic rotation of intact actin stress fibers in fibroblasts; (2) lamellipodia activity and actin polymerization in fibroblasts; (3) active axonal contraction in Drosophila embryo motor neurons. The novel platform may serve as an important tool in studying the mechanoresponse of cells and tissues, including whole embryos.
Copyright 2010 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures









References
-
- Hasegawa S, Sato S, Saito S, Suzuki Y, Brunette DM. Mechanical stretching increases the number of cultured bone cells synthesizing dna and alters their pattern of protein synthesis. Calcif Tissue Int. 1985 Jul;37(4):431–436. - PubMed
-
- Engler Adam J, Sen S, Lee Sweeney H, Discher Dennis E. Matrix elasticity directs stem cell lineage specification. Cell. 2006 Aug;126(4):677–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

