Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage
- PMID: 20189179
- PMCID: PMC2900255
- DOI: 10.1016/j.jbiomech.2010.01.021
Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage
Abstract
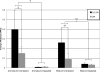
This study presents direct experimental evidence for assessing the electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage. Immature and mature bovine cartilage samples were tested in unconfined compression and their depth-dependent equilibrium compressive modulus was determined using strain measurements with digital image correlation analysis. The electrostatic contribution was assessed by testing samples in isotonic and hypertonic saline; the combined contribution was assessed by testing untreated and proteoglycan-depleted samples. Though it is well recognized that proteoglycans contribute significantly to the compressive stiffness of cartilage, results demonstrate that the combined electrostatic and non-electrostatic contributions may add up to more than 98% of the modulus, a magnitude not previously appreciated. Of this contribution, about two thirds arises from electrostatic effects. The compressive modulus of the proteoglycan-depleted cartilage matrix may be as low as 3kPa, representing less than 2% of the normal tissue modulus; experimental evidence also confirms that the collagen matrix in digested cartilage may buckle under compressive strains, resulting in crimping patterns. Thus, it is reasonable to model the collagen as a fibrillar matrix that can sustain only tension. This study also demonstrates that residual stresses in cartilage do not arise exclusively from proteoglycans, since cartilage remains curled relative to its in situ geometry even after proteoglycan depletion. These increased insights on the structure-function relationships of cartilage can lead to improved constitutive models and a better understanding of the response of cartilage to physiological loading conditions.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures







References
-
- Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2007 - PubMed
-
- Bader DL, Kempson GE. The short-term compressive properties of adult human articular cartilage. Biomed Mater Eng. 1994;4:245–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

