Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial
- PMID: 20189239
- PMCID: PMC2849002
- DOI: 10.1016/S0140-6736(10)60239-5
Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial
Erratum in
- Lancet. 2010 Jul 10;376(9735):90. Nasser, H-C [corrected to Nahser, H-C]
Abstract
Background: Stents are an alternative treatment to carotid endarterectomy for symptomatic carotid stenosis, but previous trials have not established equivalent safety and efficacy. We compared the safety of carotid artery stenting with that of carotid endarterectomy.
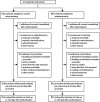
Methods: The International Carotid Stenting Study (ICSS) is a multicentre, international, randomised controlled trial with blinded adjudication of outcomes. Patients with recently symptomatic carotid artery stenosis were randomly assigned in a 1:1 ratio to receive carotid artery stenting or carotid endarterectomy. Randomisation was by telephone call or fax to a central computerised service and was stratified by centre with minimisation for sex, age, contralateral occlusion, and side of the randomised artery. Patients and investigators were not masked to treatment assignment. Patients were followed up by independent clinicians not directly involved in delivering the randomised treatment. The primary outcome measure of the trial is the 3-year rate of fatal or disabling stroke in any territory, which has not been analysed yet. The main outcome measure for the interim safety analysis was the 120-day rate of stroke, death, or procedural myocardial infarction. Analysis was by intention to treat (ITT). This study is registered, number ISRCTN25337470.
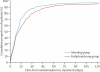
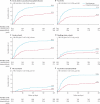
Findings: The trial enrolled 1713 patients (stenting group, n=855; endarterectomy group, n=858). Two patients in the stenting group and one in the endarterectomy group withdrew immediately after randomisation, and were not included in the ITT analysis. Between randomisation and 120 days, there were 34 (Kaplan-Meier estimate 4.0%) events of disabling stroke or death in the stenting group compared with 27 (3.2%) events in the endarterectomy group (hazard ratio [HR] 1.28, 95% CI 0.77-2.11). The incidence of stroke, death, or procedural myocardial infarction was 8.5% in the stenting group compared with 5.2% in the endarterectomy group (72 vs 44 events; HR 1.69, 1.16-2.45, p=0.006). Risks of any stroke (65 vs 35 events; HR 1.92, 1.27-2.89) and all-cause death (19 vs seven events; HR 2.76, 1.16-6.56) were higher in the stenting group than in the endarterectomy group. Three procedural myocardial infarctions were recorded in the stenting group, all of which were fatal, compared with four, all non-fatal, in the endarterectomy group. There was one event of cranial nerve palsy in the stenting group compared with 45 in the endarterectomy group. There were also fewer haematomas of any severity in the stenting group than in the endarterectomy group (31 vs 50 events; p=0.0197).
Interpretation: Completion of long-term follow-up is needed to establish the efficacy of carotid artery stenting compared with endarterectomy. In the meantime, carotid endarterectomy should remain the treatment of choice for patients suitable for surgery.
Funding: Medical Research Council, the Stroke Association, Sanofi-Synthélabo, European Union.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

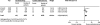
Comment in
-
Carotid stenting: more risky than endarterectomy and often no better than medical treatment alone.Lancet. 2010 Mar 20;375(9719):957-9. doi: 10.1016/S0140-6736(10)60404-7. Lancet. 2010. PMID: 20304225 No abstract available.
-
Stroke: Early trial results favor surgery over stenting for symptomatic carotid artery stenosis.Nat Rev Neurol. 2010 May;6(5):237. doi: 10.1038/nrneurol.2010.39. Nat Rev Neurol. 2010. PMID: 20455281 No abstract available.
-
[Quo vadis carotid artery stenting?].Radiologe. 2010 Aug;50(8):651-2. doi: 10.1007/s00117-010-2035-4. Radiologe. 2010. PMID: 20577867 German. No abstract available.
-
Carotid artery stenting versus endarterectomy for carotid stenosis.Lancet. 2010 Jul 31;376(9738):327; author reply 327-8. doi: 10.1016/S0140-6736(10)61177-4. Lancet. 2010. PMID: 20674712 No abstract available.
-
Carotid artery stenting versus endarterectomy for carotid stenosis.Lancet. 2010 Jul 31;376(9738):327; author reply 327-8. doi: 10.1016/S0140-6736(10)61176-2. Lancet. 2010. PMID: 20674713 No abstract available.
References
-
- European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. - PubMed
-
- Barnett HJ, Taylor DW, Eliasziw M. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. - PubMed
-
- Rothwell PM, Eliasziw M, Gutnikov SA, for the Carotid Endarterectomy Trialists' Collaboration Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107–116. - PubMed
-
- CAVATAS investigators Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–1737. - PubMed
-
- Mas JL, Chatellier G, Beyssen B. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

