Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase
- PMID: 20189400
- PMCID: PMC2841358
- DOI: 10.1016/j.bmc.2010.01.063
Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase
Abstract
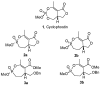
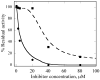
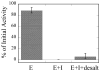
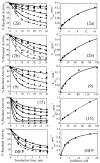
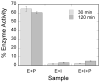
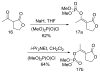
Two new monocyclic analogs of the natural AChE inhibitor cyclophostin and two exocyclic enol phosphates were synthesized. The potencies and mechanisms of inhibition of the bicyclic and monocyclic enol phosphonates and the exocyclic enol phosphates toward human AChE are examined. One diastereoisomer of the bicyclic phosphonate exhibits an IC(50) of 3 microM. Potency is only preserved when the cyclic enol phosphonate is intact and conjugated to an ester. Kinetic analysis indicates both a binding and a slow inactivation step for all active compounds. Mass spectrometric analysis indicates that the active site Ser is indeed phosphorylated by the bicyclic phosphonate.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
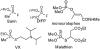

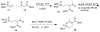
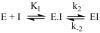
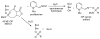
Similar articles
-
Synthesis and comparison of the biological activity of monocyclic phosphonate, difluorophosphonate and phosphate analogs of the natural AChE inhibitor cyclophostin.Bioorg Med Chem. 2015 Dec 15;23(24):7529-34. doi: 10.1016/j.bmc.2015.10.044. Epub 2015 Oct 30. Bioorg Med Chem. 2015. PMID: 26585276
-
The first total synthesis of (±)-cyclophostin and (±)-cyclipostin P: inhibitors of the serine hydrolases acetyl cholinesterase and hormone sensitive lipase.Org Lett. 2011 Jun 17;13(12):3094-7. doi: 10.1021/ol200991x. Epub 2011 May 17. Org Lett. 2011. PMID: 21591624 Free PMC article.
-
Synthesis and biological evaluation of a phosphonate analog of the natural acetyl cholinesterase inhibitor cyclophostin.J Org Chem. 2008 Nov 7;73(21):8386-91. doi: 10.1021/jo801453v. Epub 2008 Sep 27. J Org Chem. 2008. PMID: 18821801 Free PMC article.
-
The Chemistry and Biology of Cyclophostin, the Cyclipostins and Related Compounds.Molecules. 2019 Jul 16;24(14):2579. doi: 10.3390/molecules24142579. Molecules. 2019. PMID: 31315184 Free PMC article. Review.
-
Reactivators of butyrylcholinesterase inhibited by organophosphorus compounds.Bioorg Chem. 2024 Sep;150:107526. doi: 10.1016/j.bioorg.2024.107526. Epub 2024 Jun 4. Bioorg Chem. 2024. PMID: 38878749 Review.
Cited by
-
Synthesis and kinetic evaluation of cyclophostin and cyclipostins phosphonate analogs as selective and potent inhibitors of microbial lipases.J Med Chem. 2012 Nov 26;55(22):10204-19. doi: 10.1021/jm301216x. Epub 2012 Nov 7. J Med Chem. 2012. PMID: 23095026 Free PMC article.
-
Design, Synthesis and Bioactivity Evaluation of Heterocycle-Containing Mono- and Bisphosphonic Acid Compounds.Molecules. 2023 Nov 9;28(22):7509. doi: 10.3390/molecules28227509. Molecules. 2023. PMID: 38005231 Free PMC article.
-
Cyclophostin and Cyclipostins analogues counteract macrolide-induced resistance mediated by erm(41) in Mycobacterium abscessus.J Biomed Sci. 2024 Dec 3;31(1):103. doi: 10.1186/s12929-024-01091-w. J Biomed Sci. 2024. PMID: 39623375 Free PMC article.
-
Drug-target residence time: critical information for lead optimization.Curr Opin Chem Biol. 2010 Aug;14(4):467-74. doi: 10.1016/j.cbpa.2010.06.176. Epub 2010 Jul 19. Curr Opin Chem Biol. 2010. PMID: 20663707 Free PMC article. Review.
-
Novel selective and irreversible mosquito acetylcholinesterase inhibitors for controlling malaria and other mosquito-borne diseases.Sci Rep. 2013;3:1068. doi: 10.1038/srep01068. Epub 2013 Jan 15. Sci Rep. 2013. PMID: 23323211 Free PMC article.
References
-
- Kidd D, Liu Y, Cravatt BF. Biochemistry. 2001;40:4005. - PubMed
-
- Worek F, Thiermann H, Szinicz L, Eyer P. Biochem Pharmacol. 2004;68:2237. - PubMed
-
- Mount C, Downton C. Nat Med. 2006;12:780. - PubMed
-
- Musial A, Bajda M, Malawska B. Curr Med Chem. 2007;14:2654. - PubMed
-
- Zhou X, Wang XB, Wang T, Kong LY. Bioorg Med Chem. 2008;16:8011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

