Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase
- PMID: 20189400
- PMCID: PMC2841358
- DOI: 10.1016/j.bmc.2010.01.063
Synthesis and kinetic analysis of some phosphonate analogs of cyclophostin as inhibitors of human acetylcholinesterase
Abstract
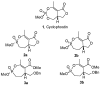
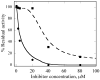
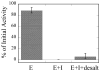
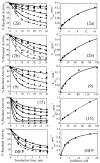
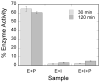
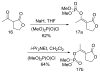
Two new monocyclic analogs of the natural AChE inhibitor cyclophostin and two exocyclic enol phosphates were synthesized. The potencies and mechanisms of inhibition of the bicyclic and monocyclic enol phosphonates and the exocyclic enol phosphates toward human AChE are examined. One diastereoisomer of the bicyclic phosphonate exhibits an IC(50) of 3 microM. Potency is only preserved when the cyclic enol phosphonate is intact and conjugated to an ester. Kinetic analysis indicates both a binding and a slow inactivation step for all active compounds. Mass spectrometric analysis indicates that the active site Ser is indeed phosphorylated by the bicyclic phosphonate.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
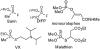

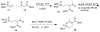
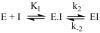
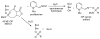
References
-
- Kidd D, Liu Y, Cravatt BF. Biochemistry. 2001;40:4005. - PubMed
-
- Worek F, Thiermann H, Szinicz L, Eyer P. Biochem Pharmacol. 2004;68:2237. - PubMed
-
- Mount C, Downton C. Nat Med. 2006;12:780. - PubMed
-
- Musial A, Bajda M, Malawska B. Curr Med Chem. 2007;14:2654. - PubMed
-
- Zhou X, Wang XB, Wang T, Kong LY. Bioorg Med Chem. 2008;16:8011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

