Age-related changes in Arc transcription and DNA methylation within the hippocampus
- PMID: 20189687
- PMCID: PMC2888808
- DOI: 10.1016/j.neurobiolaging.2010.01.009
Age-related changes in Arc transcription and DNA methylation within the hippocampus
Abstract
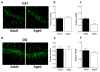
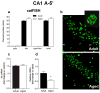
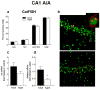
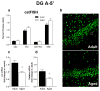
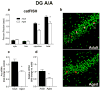
The transcription of genes that support memory processes are likely to be impacted by the normal aging process. Because Arc is necessary for memory consolidation and enduring synaptic plasticity, we examined Arc transcription within the aged hippocampus. Here, we report that Arc transcription is reduced within the aged hippocampus compared to the adult hippocampus during both "off line" periods of rest, and following spatial behavior. This reduction is observed within ensembles of CA1 "place cells", which make less mRNA per cell, and in the dentate gyrus (DG) where fewer granule cells are activated by behavior. In addition, we present data suggesting that aberrant changes in methylation of the Arc gene may be responsible for age-related decreases in Arc transcription within CA1 and the DG. Given that Arc is necessary for normal memory function, these subregion-specific epigenetic and transcriptional changes may result in less efficient memory storage and retrieval during aging.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

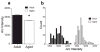

References
-
- Barnes CA, McNaughton BL. Neurophysiological comparison of dendritic cable properties in adolescent, middle-aged, and senescent rats. Exp Aging Res. 1979;5:195–206. - PubMed
-
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. - PubMed
-
- Bostock E, Muller RU, Kubie JL. Experience-dependent modifications of hippocampal place cell firing. Hippocampus. 1991;1:193–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

