Production of recombinant proteins from protozoan parasites
- PMID: 20189877
- PMCID: PMC2862126
- DOI: 10.1016/j.pt.2010.02.004
Production of recombinant proteins from protozoan parasites
Abstract
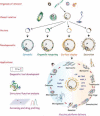
Although the past decade has witnessed sequencing from an increasing number of parasites, modern high-throughput DNA sequencing technologies have the potential to generate complete genome sequences at even higher rates. Along with the discovery of genes that might constitute potential targets for chemotherapy or vaccination, the need for novel protein expression platforms has become a pressing matter. In addition to reviewing the advantages and limitations of the currently available and emerging expression systems, we discuss novel approaches that could overcome current limitations, including the 'pseudoparasite' concept, an expression platform in which the choice of the surrogate organism is based on its phylogenetic affinity to the target parasite, while taking advantage of the whole engineered organism as a vaccination adjuvant.
Figures

Similar articles
-
Transient Expression of Plasmodium berghei MSP8 and HAP2 in the Marine Protozoan Parasite Perkinsus marinus.J Parasitol. 2017 Feb;103(1):118-122. doi: 10.1645/16-88. Epub 2016 Oct 10. J Parasitol. 2017. PMID: 27723436 Free PMC article.
-
Cell-Free Protein Synthesis: Pros and Cons of Prokaryotic and Eukaryotic Systems.Chembiochem. 2015 Nov;16(17):2420-31. doi: 10.1002/cbic.201500340. Epub 2015 Oct 19. Chembiochem. 2015. PMID: 26478227 Free PMC article. Review.
-
Antibody profiles to wheat germ cell-free system synthesized Plasmodium falciparum proteins correlate with protection from symptomatic malaria in Uganda.Vaccine. 2017 Feb 7;35(6):873-881. doi: 10.1016/j.vaccine.2017.01.001. Epub 2017 Jan 12. Vaccine. 2017. PMID: 28089547
-
[Expression and purification of Toxoplasma gondii GRA4 gene in prokaryotic system].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005 Dec 30;23(6):419-23. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005. PMID: 16566210 Chinese.
-
Untranslated regions of mRNA and their role in regulation of gene expression in protozoan parasites.J Biosci. 2017 Mar;42(1):189-207. doi: 10.1007/s12038-016-9660-7. J Biosci. 2017. PMID: 28229978 Review.
Cited by
-
HeLa Based Cell Free Expression Systems for Expression of Plasmodium Rhoptry Proteins.J Vis Exp. 2015 Jun 10;(100):e52772. doi: 10.3791/52772. J Vis Exp. 2015. PMID: 26131624 Free PMC article.
-
Insights into Peptidyl-Prolyl cis-trans Isomerases from Clinically Important Protozoans: From Structure to Potential Biotechnological Applications.Pathogens. 2024 Jul 31;13(8):644. doi: 10.3390/pathogens13080644. Pathogens. 2024. PMID: 39204244 Free PMC article. Review.
-
Leishmania infantum recombinant kinesin degenerated derived repeat (rKDDR): A novel potential antigen for serodiagnosis of visceral leishmaniasis.PLoS One. 2019 Jan 31;14(1):e0211719. doi: 10.1371/journal.pone.0211719. eCollection 2019. PLoS One. 2019. PMID: 30703138 Free PMC article.
-
An Agar-Based Method for Plating Marine Protozoan Parasites of the Genus Perkinsus.PLoS One. 2016 May 5;11(5):e0155015. doi: 10.1371/journal.pone.0155015. eCollection 2016. PLoS One. 2016. PMID: 27149378 Free PMC article.
-
Functional Comparison of Blood-Stage Plasmodium falciparum Malaria Vaccine Candidate Antigens.Front Immunol. 2019 Jun 4;10:1254. doi: 10.3389/fimmu.2019.01254. eCollection 2019. Front Immunol. 2019. PMID: 31214195 Free PMC article.
References
-
- de Azevedo WF, Jr., Soares MB. Selection of targets for drug development against protozoan parasites. Curr. Drug Targets. 2009;10:193–201. - PubMed
-
- Coombs A. The sequencing shakeup. Nat. Biotechnol. 2008;26:1109–1112. - PubMed
-
- Seitzer U, et al. Evaluation of Theileria annulata recombinant immunodominant proteins for the development of ELISA. Transbound. Emerg. Dis. 2008;55:244–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

