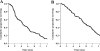Fixed-dose-rate gemcitabine: a viable first-line treatment option for advanced pancreatic and biliary tract cancer
- PMID: 20189980
- PMCID: PMC3227937
- DOI: 10.1634/theoncologist.2008-0135
Fixed-dose-rate gemcitabine: a viable first-line treatment option for advanced pancreatic and biliary tract cancer
Abstract
Background: We have already reported on fixed-dose-rate gemcitabine (FDR-Gem) in advanced, inoperable pancreatic ductal adenocarcinoma (PDAC) and biliary tract cancer (BTC) in the context of a formal phase II study; building on that experience, we have now expanded the study to reach a cumulative accrual of 106 patients.
Methods: One hundred six patients (PDAC/BTC, 75/31) were treated with weekly FDR-Gem (1,000 mg/m(2) infused at 10 mg/m(2) per minute). Patient characteristics included: male-to-female ratio, 0.83; median age, 63 years (range, 28-82); metastatic disease in 66% of patients; and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0-1 in 81% of patients.
Results: The median and total number of treatment weeks delivered were 8 (range, 2-22) and 1,154, respectively. Thirteen percent of patients achieved an objective response, 42% experienced a positive clinical benefit response, and 54% achieved a >50% reduction in serum cancer antigen (CA)19.9 levels. The median progression-free survival (PFS) and overall survival (OS) times for the entire population were 4.4 months (95% confidence interval [CI], 3.5-5.1 months) and 7.7 months (95% CI, 6.3-8.8 months), respectively, with 20% of patients alive at 1 year. On multivariate analysis, a CA19.9 reduction >50% and baseline ECOG PS score of 0 were the only independent predictors of PFS and OS, respectively. Treatment was well tolerated, with grade 3-4 neutropenia in 47 of 1,154 treatment weeks (4.1%), and grade 3 anemia and thrombocytopenia in 8 of 1,154 (0.7%) and 16 of 1,154 (1.4%) treatment weeks, respectively.
Conclusions: Currently available evidence, including this updated analysis, supports the use of FDR-Gem as a first-line option in advanced PDAC, and possibly in BTC, patients and prompts the continued evaluation of this approach in combination regimens.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers.
Figures
Similar articles
-
Fixed dose-rate gemcitabine infusion as first-line treatment for advanced-stage carcinoma of the pancreas and biliary tree.Cancer. 2005 Sep 15;104(6):1237-45. doi: 10.1002/cncr.21286. Cancer. 2005. PMID: 16078261 Clinical Trial.
-
A Multicenter Phase II Study of Gemcitabine plus S-1 Chemotherapy for Advanced Biliary Tract Cancer.Anticancer Res. 2017 Feb;37(2):909-914. doi: 10.21873/anticanres.11398. Anticancer Res. 2017. PMID: 28179351 Clinical Trial.
-
Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group.J Clin Oncol. 2009 Aug 10;27(23):3778-85. doi: 10.1200/JCO.2008.20.9007. Epub 2009 Jul 6. J Clin Oncol. 2009. PMID: 19581537 Free PMC article. Clinical Trial.
-
Efficacy and safety of gemcitabine-based chemotherapies in biliary tract cancer: a meta-analysis.World J Gastroenterol. 2014 Dec 21;20(47):18001-12. doi: 10.3748/wjg.v20.i47.18001. World J Gastroenterol. 2014. PMID: 25548500 Free PMC article. Review.
-
Chemotherapy with gemcitabine in advanced biliary tract carcinoma.Rev Recent Clin Trials. 2008 Jan;3(1):70-8. doi: 10.2174/157488708783330512. Rev Recent Clin Trials. 2008. PMID: 18474016 Review.
Cited by
-
First-line erlotinib and fixed dose-rate gemcitabine for advanced pancreatic cancer.World J Gastroenterol. 2013 Jul 28;19(28):4511-9. doi: 10.3748/wjg.v19.i28.4511. World J Gastroenterol. 2013. PMID: 23901226 Free PMC article. Clinical Trial.
-
Metastatic pancreatic cancer: Is there a light at the end of the tunnel?World J Gastroenterol. 2015 Apr 28;21(16):4788-801. doi: 10.3748/wjg.v21.i16.4788. World J Gastroenterol. 2015. PMID: 25944992 Free PMC article. Review.
-
Extracellular Matrix Composition Modulates the Responsiveness of Differentiated and Stem Pancreatic Cancer Cells to Lipophilic Derivate of Gemcitabine.Int J Mol Sci. 2020 Dec 22;22(1):29. doi: 10.3390/ijms22010029. Int J Mol Sci. 2020. PMID: 33375106 Free PMC article.
-
Developments in metastatic pancreatic cancer: is gemcitabine still the standard?World J Gastroenterol. 2012 Feb 28;18(8):736-45. doi: 10.3748/wjg.v18.i8.736. World J Gastroenterol. 2012. PMID: 22371633 Free PMC article. Review.
References
-
- Veltkamp SA, Beijnen JH, Schellens JH. Prolonged versus standard gemcitabine infusion: Translation of molecular pharmacology to new treatment strategy. The Oncologist. 2008;13:261–276. - PubMed
-
- Burris H, 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: Targeting the epidermal growth factor and vascular endothelial growth factor pathways. The Oncologist. 2008;13:289–298. - PubMed
-
- Gelibter A, Malaguti P, Di Cosimo S, et al. Fixed dose-rate gemcitabine infusion as first-line treatment for advanced-stage carcinoma of the pancreas and biliary tree. Cancer. 2005;104:1237–1245. - PubMed
-
- Storniolo AM, Enas NH, Brown CA, et al. An investigational new drug treatment program for patients with gemcitabine: Results for over 3000 patients with pancreatic carcinoma. Cancer. 1999;85:1261–1268. - PubMed
-
- Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials